(a) The cis-and trans-isomers of [PtCl 2 (NH 3 )(NO 2 )] are prepared by reaction...
Question:
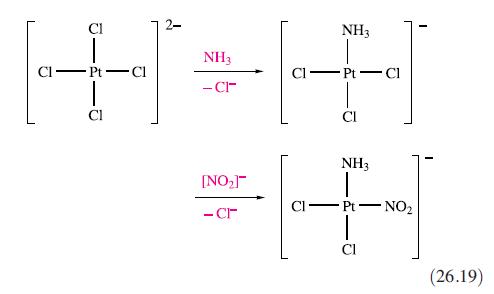
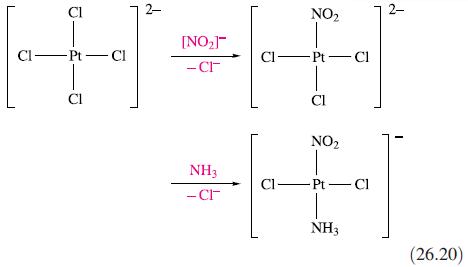
(a) The cis-and trans-isomers of [PtCl2(NH3)(NO2)]− are prepared by reaction sequences 26.19 and 26.20 respectively. Rationalize the observed differences in products in these routes.
(b) Suggest the products of the reaction of [PtCl4]2− with PEt3.
Transcribed Image Text:
CI U Pt-Cl Cl ĭ NH3 -CI [NO₂] - cr CI NH3 Pt NH3 - Cl Pt-NO₂ (26.19)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a To rationalize the observed differences in products between reaction sequences 2619 and 2620 we ne...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Your firm has selected you to develop and assess the control risk over shipping and billing functions of ABC Company. The audit manager wishes to rely on control risk assessment at a low level. In...
-
The P/E ratio on the S&P 500 Index for 1998and 1999 was 30 or higher. Other things equal,would this indicate a good time to buy stocks fora multi-year holding period, or not?
-
Write structures for the cis and trans isomers of (a) 1,2-dichlorocyclopentane and (b) 1,3-dibromocyclobutane. (c) Are cis-trans isomers possible for 1,1-dibromocyclobutane?
-
Which of the following is not an example of supervised machine learning? O Clustering O Deep learning O Decision Tree Linear Regression
-
Since Smart Enterprises inaugurated participative budgeting 10 years ago, everyone in the organizationfrom maintenance personnel to the presidents staffhas had a voice in the budgeting process. Until...
-
Dom, the manager of a fishery, determines that t weeks after 300 fish of a particular species are released into a pond, the average weight of an individual fish (in pounds) for the first 10 weeks...
-
How would you describe the findings of at least one important piece of research on achieving high-performance in public organizations? What are its implications for the practice of public management?...
-
Winnebago Industries manufactures motor homes. Adapted versions of the companys balance sheets (in $000) follow. Required Prepare a common-size balance sheet for each fiscal year, rounding all...
-
Imperial Jewelers manufactures and sells a gold bracelet for $403.00. The company's accounting system says that the unit product cost for this bracelet is $261.00 as shown below: Direct materials...
-
(a) Suggest a mechanism for the reaction: (b) If the intermediate in your mechanism is sufficiently long-lived, what complication might arise? trans-[PtLCl] + Y trans-[PtLCIY]+ + CI CI
-
Under pseudo-first order conditions, the variation of k obs with [py] for reaction of square planar [Rh(cod)(PPh 3 ) 2 ] + (2 10 4 mol dm 3 ; cod= 24:22) with pyridine is as follows: Show that the...
-
How could a business selling firewood could create a more attractive economic value?
-
"The initial speed with which a ball is thrown is doubled, with the angle of projection fixed. Is the maximum height to which the ball rises doubled?" Now, let's say you are also allowed to change...
-
Wally Working Co. emiti bonos con una tasa de inters nominal (contratada) de 15%, por un valor ominal de $80,000, con un vencimiento de 5 anios. Cuando emiti los bonos, la tasa de inters del mercado...
-
Using the Central Limit Theorem. In Exercises 5-8, assume that the amounts of weight that male college students gain during their freshman year are normally distributed with a mean of 1.2 kg and a...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Please help Calculating NPV and IRR Businesses use NPV and IRR to determine whether a project will add - value for shareholders. After watching the CFA Level I Corporate Finance video, answer the...
-
What is meant by non operating revenues and gains?
-
How does the organizational structure of an MNC influence its strategy implementation?
-
Explain why the IUPAC name of a compound will never end with the suffix -1-one.
-
For each pair of the following compounds, identify which compound would be expected to react more rapidly with a nucleophile: (a) (b) H.
-
Draw the products of each Wittig reaction below. If two stereoisomers are possible, draw both stereoisomers: (a) (b) Ph Ph Ph-P Ph Ph. Ph-P Ph, Ph Ph
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


