(a) The value of eff for [CoF 6 ] 3 is 5.63 B . Explain...
Question:
(a) The value of μeff for [CoF6]3− is 5.63 μB. Explain why this value does not agree with the value for μ calculated from the spin-only formula.
(b) By using a simple MO approach, rationalize why one-electron oxidation of the bridging ligand in [(CN)5CoOOCo(CN)5]6− leads to a shortening of the O—O bond.
(c) Salts of which of the following complex ions might be expected to be formed as racemates:
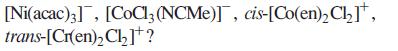
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





