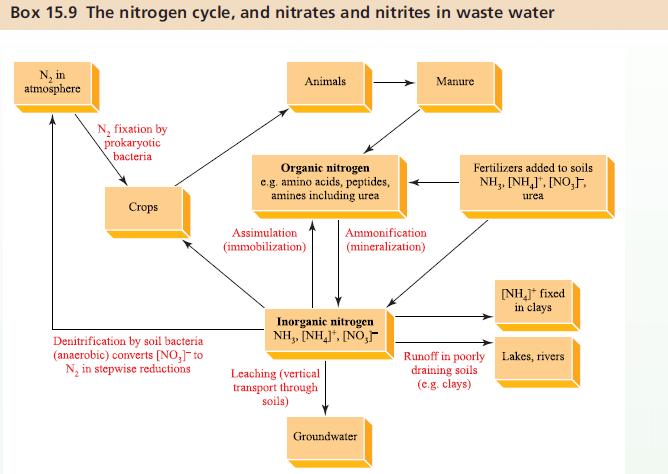
Box 15.9 deals with the nitrogen cycle and the removal of nitrates and nitrites from waste water.
Question:
Box 15.9 deals with the nitrogen cycle and the removal of nitrates and nitrites from waste water.
(a) Urea is used to reduce [NO2]− to N2. Write a balanced equation for the reaction of HNO2 with urea.
(b) Sulfamic acid is also used to reduce [NO2]− to N2 during water treatment. Give an equation for this reaction.
(c) Nitrites can be removed using H2O2, [OCl]− or HOCl as oxidants. How does the reduction potential for the following process depend on pH?
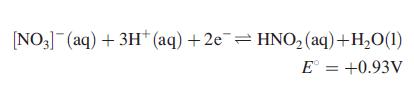
Suggest products for the reduction of H2O2 and HOCl in acidic, aqueous solution. Give equations for the reactions of H2O2 with [NO2]− in acidic solution, and for [OCl]− with [NO2]− in alkaline solution.
Box 15.9.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





