By considering the reactions 8E(g) 4E 2 (g) and 8E(g) E 8 (g) for E
Question:
By considering the reactions 8E(g) → 4E2(g) and 8E(g) → E8(g) for E = O and E = S, show that the formation of diatomic molecules is favoured for oxygen, whereas ring formation is favoured for sulfur. [Data: see Table 16.2.]
Table 16.2.
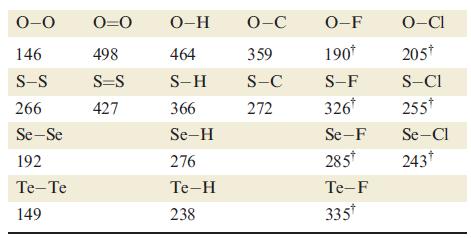
Transcribed Image Text:
0-0 146 S-S 266 Se-Se 192 Te-Te 149 0=0 498 S=S 427 O-H 464 S-H 366 Se-H 276 Te-H 238 O-C 359 S-C 272 O-F 190* S-F 326* Se-F 285* Te-F 335* O-CI 205* S-CI 255* Se-Cl 243†
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
To determine whether the formation of diatomic molecules or ring formation is favored for oxygen O and sulfur S we need to compare the enthalpy changes H for the two reactions 8Eg 4E2g and 8Eg E8g for E O and E S Lets consider the data from Table 162 for oxygen O and sulfur S For oxygen O Enthalpy change ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the runs test to determine whether the sample is random. Let alpha be.05. 2 2 2 2 2 1 2 2 2 1 1 2 2 2
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
John works in a factory as a Quality Expert and is responsible for doing quality product testing before the finished products are sent to the customers. John takes a sample of 10 from a batch of 1000...
-
Under a perpetual inventory system, a company should know the quantity and price of its inventory at any moment in time. Given this, why do companies that use a perpetual inventory system still take...
-
For Problems sketch a possible graph of y = f(x), using the given information about the derivatives y' = f'(x) and y'' = f''(x). Assume that the function is defined and continuous for all real x. y =...
-
What are incomparable outcomes in the context of international performance evaluations? Give a suitable example. LO.1
-
The Biological Insect Control Corporation (BICC) has hired you as a consultant to evaluate the NPV of its proposed toad ranch. BICC plans to breed toads and sell them as ecologically desirable insect...
-
Problem 1 8 - 5 2 ( LO . 7 ) Refer to Example 4 2 in text Section 1 8 - 5 f . Albert owns 1 0 0 % of A Corporation, Betty is the sole proprietor of B Company, and Cai is the sole proprietor of C...
-
(a) Use the values of E for reactions 16.32 and 16.33 to show that H 2 O 2 is thermodynamically unstable with respect to decomposition into H 2 O and O 2 . (b) 20 Volume H 2 O 2 is so called because...
-
Polyphosphazenes are an important class of inorganic macromolecule and have many commercial applications, e.g. fire retardants, elastomers, fuel cell membranes, biomedical applications. The scheme...
-
Can 2,4-pentanedione undergo an intramolecular aldol addition? If so, why? If not, why not?
-
there are some solbeed with direct materials. this one says direct labor. any help would be appreciated, ive been stuck Chapter 9 Homework Save 1.5 6 H 305 Parker Plastic, Incorporated, manufactures...
-
Give examples of applications where pumps might be connected in series. Give examples of applications where pumps might be connected in parallel. Drawing on the conclusions of earlier exercises,...
-
a truck company has 2 trucks, which are hired out day by day. The average number of trucks hired on a day follows a distribution with mean 1 . 5 . Identify the distribution and then find the...
-
Designand drive selectionfor a hydrostaticapplication.Choose anypropelledequipmentwithopen or closedloop HST. Includethepayloadand/or anymachinefunctionrequirementsfor the mobileequipment.A sketch...
-
A two stage air compressor with ideal intercooler pressure and perfect intercooling (what does this mean?) compresses air from 1 bar to 16 bar at the rate of 5 m3/min. Mechanical efficiency of the...
-
What came in-between the Declaration of Independence and the adoption of the US Constitution?
-
Find the radius of convergence of? 1.2.3 1.3.5 (2n-1) r2n+1 -1
-
Predict the shape of the doubly chlorine-bridged I 2 Cl 6 molecule by using the VSEPR model, and assign the point group.
-
(a) Use the VSEPR model to predict the probable shapes of [IF 6 ] + and IF 7 . (b) Give a plausible chemical equation for the preparation of [IF 6 ][SbF 6 ].
-
Sketch all the isomers of the complexes [CrCl 4 F 2 ] 3 and [CrCl 3 F 3 ] 3 . Indicate how many fluorine environments would be indicated in the 19 F-NMR spectrum of each isomer.
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


