Calculate the equilibrium constant of the reaction from the standard potentials Aut(aq) + 2 CN- (aq) [Au(CN)](aq)
Question:
Calculate the equilibrium constant of the reaction
![]()
from the standard potentials
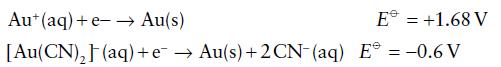
Transcribed Image Text:
Aut(aq) + 2 CN- (aq) [Au(CN)₂](aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To calculate the equilibrium constant Keq for the given reaction we can use the Nernst equation The Nernst equation relates the standard electrode pot...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Use electrode potentials to calculate the equilibrium constant at 25oC for the reaction See Appendix I for data. cio4(aq) + cio,-(aq)-2010,-(aq)
-
From the following reduction potentials (a) Calculate the equilibrium constant for .I2 (aq) + I- I-3 (b) Calculate the equilibrium constant for I2 (aq) + I- I-3 (c) Calculate the solubility (g/L) of...
-
Leno Company used regression analysis to predict the annual cost of indirect materials. The results were as follows: Indirect Materials Cost Explained by Units Produced Constant 14,885 Standard error...
-
A voltage V is applied to a dc electric motor. The armature winding resistance is equal to R. At what value of current flowing through the winding will the useful power of the motor be the highest?...
-
Multiple-Choice Questions 1. Microsoft found that instead of producing a DVD player and a gaming system separately, it is cheaper to incorporate DVD playing capabilities in their new version of the...
-
A liquid macrofluid reacts according to A R as it flows through a vessel. Find the conversion of A for the flow patterns CA04 mol/liter -TA = k k = 1 mol/liter min E, min-1 3 Area = 1 1, min
-
List the factors that affect the consumer decision process.
-
State the effect (cash receipt or payment and amount) of each of the following transactions, considered individually, on cash flows: a. Sold equipment with a book value of $65,000 for $83,000. b....
-
Required a . Assume that Stuart has decided to accept one of the two jobs. Fill in the information relevant to selecting one job versus the other. Recommend which job to accept. b . Assume that Job A...
-
From the following Latimer diagram (which does not correspond to standard conditions), calculate the value of E for the reaction 2HO 2 (aq) O 2 (g) + H 2 O 2 (aq). Comment on the thermodynamic...
-
In their article Enzymes and bio-inspired electrocatalysts in solar fuel devices (Energy Environ. Sci., 2012, 5, 7470), Woolerton et al. use a generic Frost diagram (Fig. 6.21) to illustrate the...
-
An experimental setup consists of a \(550-\mathrm{nm}\) laser, a screen at a distance of \(0.50 \mathrm{~m}\), and an adjustable-width single slit. At what slit width is the width of the central...
-
The following information was obtained from the records of Shae Inc.: Merchandise inventory $ 88,000 Notes payable (long-term) 100,000 Net sales 300,000 Buildings and equipment 168,000 Selling,...
-
Absent Clothing Company Savita Kapur, CEO, founded Absent Clothing Company (ACC) in 2005. ACC sells practical athletic wear to service the yoga and pilates market. Savita originally created ACC with...
-
Find the indicated area under the curve of the standard normal distribution; then convert it to a percentage and fill in the blank. About % of the area is between z = - 3.5 and z = 3.5 (or...
-
EM 605 Spring 2021 Midterm Exam 3/17/2021 The linear programming problem whose output follows is used to determine how many bottles of Hell-bound red nail polish (x1), Blood red nail polish (x2),...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Refer to the Significance (December 2015) study estimating the number of U.S. deaths attributable to nitrous oxide pollution (NOx) produced from illegal VW vehicles, Exercise 2.30 (p. 61). The...
-
Vince, Inc. has developed and patented a new laser disc reading device that will be marketed internationally. Which of the following factors should Vince consider in pricing the device? I. Quality of...
-
Suggest possible solid state precursors for the formation of the following compounds by pyrolysis reactions: (a) BiCaVO 5 ; (b) The Mo(VI) oxide CuMo 2 YO 8 ; (c) Li 3 InO 3 ; (d) Ru 2 Y 2 O 7 .
-
(a) Describe the relationship between the structures of MgB 2 and graphite. (b) How does the electronic structure of MgB 2 differ from that of graphite, and how does this affect the properties of the...
-
(a) Describe the layered structure of FeSe. (b) How are the structures of NaFeAs and LaOFeAs related to that of FeSe? (c) Whereas LaOFeAs is not superconducting, LaO 1x F x FeAs is a superconductor...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


