Describe the bonding in Ga 2 H 6 and Ga 2 Cl 6 , both of which
Question:
Describe the bonding in Ga2H6 and Ga2Cl6, both of which have structures of the type shown in 13.50.
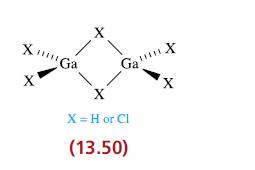
Transcribed Image Text:
XmGa X Ga X X = H or Cl (13.50) X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The compounds Ga2H6 digallane and Ga2Cl6 digallium hexachloride both exhibit unique bonding patterns ...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Describe the bonding in the CO32- ion using the LE model. How would the molecular orbital model describe the bonding in this species?
-
Describe the bonding in the O3 molecule and the NO2- ion using the LE model. How would the molecular orbital model describe the bonding in these two species?
-
The space-filling models of ethane and ethanol are shown below. Use the LE model to describe the bonding in ethane and ethanol. Ethane Ethanol (C2Ha)CH,OH) 0
-
The two roots of a quadratic equation ax 2 + bx + c = 0 can be obtained using the following formula: b 2 - 4ac is called the discriminant of the quadratic equation. If it is positive, the equation...
-
Explain how a gain or loss on disposal is handled in a capital-budgeting analysis.
-
In Exercises (a) Find the point on the curve at which the curvature K is a maximum (b) Find the limit of K as x . y = (x - 1) + 3
-
What is escalation clause?
-
The Southern Rail Company ships coal by rail from three coal mines to meet the demand requirements of four coal depots. The following table shows the distances from the mines to the various depots...
-
Determine the amount of cash paid to suppliers for each of the four Independent situations below. Situation Cash paid to suppliers 1 2 Cost of goods sold $ 200,000 $ 200,000 300,000 300,000 Inventory...
-
Suggest explanations for the following facts. (a) Na[BH 4 ] is very much less rapidly hydrolysed by H 2 O than is Na[AlH 4 ]. (b) The rate of hydrolysis of B 2 H 6 by water vapour is given by the...
-
(a) One gallium-containing product, A, was obtained from the following reaction, carried out in Et 2 O solvent: The room temperature, solution 1 H NMR spectrum of A showed the following signals: ...
-
On January 1, 2024, the Apex Company exchanged some shares of common stock it had been holding as an investment for a note receivable. The note principal plus interest is due on January 1, 2025. The...
-
int rFibNum(int a, int b, int n) { if(n == 1) return a; else if( n == 2) return b; else return rFibNum(a,b, n-1) + rFibNum(a, b, n-2); } In the code above; a) how many base cases are there? b) what...
-
Watch the Super Nanny (i.e., Jo Frost) episode "The Orm Family" (Season 1, Episode 3) and answer the following questions. Unless otherwise specified, your answers should focus on Declan (the 3 year...
-
2. Suppose Ford officials were asked to justify their decision. What moral principles do you think they would invoke? Assess Ford's handling of the Pinto from the perspective of each of the moral...
-
2. You have been asked to design the proto-type of an Automatic Grocery Vending Machine 10 (AGVM) for the super store. Automatic Grocery Vending Machine (AGVM) is a machine where different types of...
-
1. What does Porter's 5 Forces analysis strategy do? 2. Do most people agree Why? or disagree with this aspect Why? Here is the reference video, https://www.youtube.com/watch?v=Dfp23xSqpdk 3. What...
-
The accounting records of Westcott Company revealed the following costs: Factory utilities $ 35,000 Wages of assembly-line personnel 170,000 Customer entertainment 45,000 Indirect materials use...
-
Separate variables and use partial fractions to solve the initial value problems in Problems 18. Use either the exact solution or a computer-generated slope field to sketch the graphs of several...
-
The perovskite structure, ABX 3 , can be described as a closepacked array of the A and X ions together, with B-type cations in octahedral holes. What proportion of octahedral holes is filled?
-
The structure of calcite (CaCO 3 ) is shown in Fig. 4.76. Describe how this structure is related to that of NaCl. Figure 4.76. CO Ca+
-
(a) Calculate the enthalpy of formation of the hypothetical compound KF 2 assuming a CaF 2 structure. Use the BornMayer equation to obtain the lattice enthalpy and estimate the radius of K 2+ by...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


