Explain why SF 6 is a very stable molecule whereas OF 6 is unknown. Make use of
Question:
Explain why SF6 is a very stable molecule whereas OF6 is unknown. Make use of the bond enthalpy data given in Table 2.7.
Table 2.7.
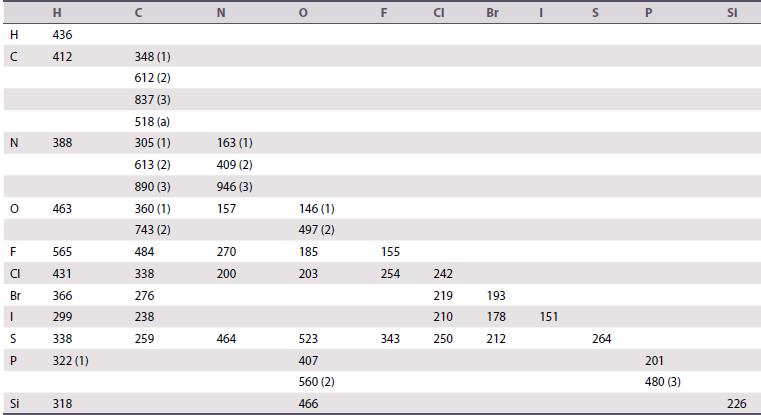
Transcribed Image Text:
H с N 0 F CI Br | S P LL Si H 436 412 388 463 565 431 366 299 338 322 (1) 318 348 (1) 612 (2) 837 (3) 518 (a) 305 (1) 613 (2) 890 (3) 360 (1) 743 (2) 484 338 276 238 259 N 163 409 (2) 946 (3) 157 270 200 (1) 464 146 (1) 497 (2) 185 203 523 407 560 (2) 466 F 155 254 343 P 242 219 210 250 Br 193 178 212 151 S 264 201 480 (3) SI 226
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
To understand why SF6 is a very stable molecule whereas OF6 is unknown we need to compare the bond e...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Main street apartments has 4 apartments rented at $1075/month.Laundry income of $5370 per year and 11 storage lockers rented at $30 per month. The vacancy rate is 1.6%. What is the effective gross...
-
Explain why borazole (sometimes called inorganic benzene) is a very stable compound. N:N borazole
-
In the conversion of a-ketoglutarate to glucose, which of the following compounds are not obligatory intermediates in this pathway? * Oxaloacetate O Fructose 1,6 bisphosphate O Malate O 1,3...
-
Route 66 Tire Co. manufactures automobile tires. Standard costs and actual costs for direct materials, direct labor, and factory overhead incurred for the manufacture of 10,000 tires were as follows:...
-
Electric resistance baseboard convectors are under consideration for heating a vacation home. The bedroom has a design heating load of 4000 Btu/hr. Identify the length of unit (in feet) needed to...
-
5. Replicate the GARCH(1,1) estimation in Example 2, using daily returns from on IBM from January 1999 to December 2003. Compare your estimates with and without the four largest returns.
-
Pomeroy Corporation owns an 80% interest in Sherer Company and a 90% interest in Tampa Company. On January 2, 2011, Tampa Company sold equipment with a book value of $600,000 to Sherer Company for...
-
Explain the regulations relative to partnerships.
-
State whether the following oxides are acidic, basic, neutral, or amphoteric: CO 2 , P 2 O 5 , SO 3 , MgO, K 2 O, Al 2 O 3 , CO.
-
Sketch the two possible geometric isomers of the octahedral [AsF 4 Cl 2 ] and explain how they could be distinguished by 19 F-NMR.
-
Distinguish between a financial statement audit, performed by external auditors, and a financial audit, performed by internal auditors.
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
1. Discuss the issue of what "waters" are subject to the Clean Water Act. 2. What are the NAAQSs and what are their primary and secondary standards?
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
Deduce possible J values for a 3 F term. What is the degeneracy of each of these J levels, and what happens when a magnetic field is applied? Sketch an energy level diagram to illustrate your answer,...
-
Which of the following ions are diamagnetic: Rationalize your answer. (a) [Co(OH)6]+, (b) [CoF, (c) [NiF, (d) [Fe(CN)6], (e) [Fe(CN)6], (f) [Mn(OH)6]+?
-
What are the limitations of the RussellSaunders coupling scheme?
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


