Explain why the standard potentials for the half-cell reactions vary with temperature in opposite directions. [Ru(NH,),P+(aq)+e [RuNH,),l(aq)
Question:
Explain why the standard potentials for the half-cell reactions
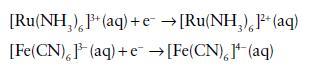
vary with temperature in opposite directions.
Transcribed Image Text:
[Ru(NH,),P+(aq)+e →[RuNH,),l(aq) [Fe(CN),(aq) +e →[Fe(CN), 1¹(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The given halfcell reactions involve the reduction of two different species namely RuNH632 and FeCN643 which can take place in solution Each halfcell ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The reaction taking place in an electrochemical cell under standard conditions is Fe 2+ (aq) + Ag + (aq) Fe 3+ (aq) + Ag(s) a. Write two half-equations for this reaction. For each, state whether...
-
Explain why the standard bank confirmation form does not identify all information about an entitys bank accounts or loans.
-
Explain why the standard deviation would likely not be a reliable measure of variability for a distribution of data that includes at least one extreme outlier?
-
Show that in the limit ??x ?? 0 and ??t ?? 0, the difference Equation (3.12) is equivalent to the differential Equation (2.5). GIVENThe difference equation for one-dimensional transient...
-
Anthony Bennett is the manufacturing production supervisor for Green Bottle Works (GBW), a company that manufactures stainless-steel water bottles. Trying to explain why he did not get the year-end...
-
Hydrogenation reactions, which involve the addition of H 2 to a molecule, are widely used in industry to transform one compound into another. For example, 1-butene (C 4 H 8 ) is converted to butane...
-
Garage rent is classified under (a) direct expense (b) indirect expense (c) standing charges (d) machine expenses
-
Listed below are several transactions that typically produce either an increase or a decrease in cash. Indicate by letter whether the cash effect of each transaction is reported on a statement of...
-
both questions pertain to the information in 11. Use the following to answer questions 11 and 12: A review of the bank statement and accounting records of the Browning Company revealed the following...
-
Using the data shown in the table, and given that the standard reduction potential for the Fe 3+ /Fe 2+ couple in aqueous solution is +0.77 V vs SHE, calculate the standard reduction potentials for...
-
An electrically conducting solution is produced when AlCl 3 is dissolved in the basic polar solvent CH 3 CN. Give formulas for the most probable conducting species and describe their formation using...
-
Determine by direct integration the moment of inertia of the shaded area with respect to the y axis. x= ky
-
Give your overall opinion . What do you think about neuromarketing? Is it usefull or is it a waste of time? Some people think this practice is "Orwellian", do you agree? Can marketers manage the...
-
Question 1. Let z= f(x,y), x = g (s, t). and ' y = h (s, t). with f, g & h all differentiable. (a) Set up an appropriate tree diagram for the of chain rule as done in this module's Use video lessons:...
-
ow do synergistic dynamics emerge within high-performance teams, and what role do diverse skill sets, complementary roles, and shared goals play in fostering collaborative innovation and collective...
-
(14%) Problem 3: The circuit shown contains a voltage source with emf & = 5.99 V, a resistor with resistance R = 135 k2, and a capacitor with capacitance C = 507 nF. When switch S is set to position...
-
1. What functions do all managers perform regularly? How do these functions apply to the three levels of management found in most organizations? 2. Identify and distinguish between the different...
-
Write a comprehensive paper on blog. Discuss the following: Description of blog What is it about? Is it hosted by an individual? Sponsored by an organization? Analysis of blog Segmentation Who is the...
-
Distinguish between the work performed by public accountants and the work performed by accountants in commerce and industry and in not-for-profit organisations.
-
(a) VO, TiO and NiO all have defect rock salt structures. Explain what this statement means. (b) In NiO, the NiO internuclear separation is 209 pm. Determine the volume of a unit cell of NiO, and its...
-
(a) How many ion-pairs are present in a unit cell of NaCl? (b) A unit cell length of 564 pm for NaCl has been determined by X-ray diffraction studies. Determine the volume of a unit cell of NaCl. (c)...
-
Explain what is meant by Frenkel and Schottky defects in an NaCl structure type.
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


