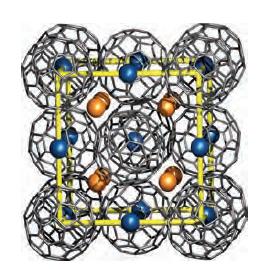
Figure 14.10 shows a unit cell of K 3 C 60 . From the structural information given,
Question:
Figure 14.10 shows a unit cell of K3C60. From the structural information given, confirm the stoichiometry of this fulleride.
Figure 14.10.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The stoichiometry of K3C60 can be confirmed from the structural information given in Figure 1410 as ...View the full answer

Answered By

Moses mwangi
With prior writing experience, be sure that I will give a great grade, If not an A+, it will be something close to this. My reviews speaks it all, Try me!!
4.80+
78+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Figure 15.23 shows a unit cell of FeSb 2 O 6 . (a) How is this unit cell related to the rutile-type structure? (b) Why can the solid state structure of FeSb 2 O 6 not be described in terms of a...
-
MgF 2 has a TiO 2 -type structure. (a) Sketch a unit cell of MgF 2 , (b) Confirm the stoichiometry of MgF 2 using the solid state structure.
-
(a) Na 3 N remained an elusive compound until 2002. Calculate a value for f H o (Na 3 N, s) using data from Appendices 8 and 10, and the following estimated values of H(298 K): Comment on whether...
-
Which of the following is not true regarding a promissory note? a. Promissory notes may not be transferred to another party by endorsement. b. Promissory notes may be sold to another party. c....
-
You are employed by a business consulting firm as an information systems specialist. You have just begun an assignment with a startup company and are discussing with the owner her need for an...
-
If automating user creation using Ansible, must you use a bash script? Are there other tools? Discuss.
-
Describe activities associated with career planning and advancement.
-
Peninsula Industries and Seaport Company, a 90 percent owned subsidiary, engage in extensive intercompany transactions involving raw materials, component parts, and completed products. Peninsula...
-
Creating and Using a Cost Formula Big Thumbs Company manufactures portable flash drives for computers. Big Thumbs incurs monthly depreciation costs of $15,400 on its plant equipment. Also, each drive...
-
Comment on each of the following observations. (a) The carbides Mg 2 C 3 and CaC 2 liberate propyne and ethyne respectively when treated with water, reaction between ThC 2 and water produces mixtures...
-
Discuss the trends in data in Table 12.4. Data from Table 12.4. Metal, M Mg Ca Sr Ba MF2 -1113 -1214 -1213 -1200 AH/kJ mol MC1 -642 -795 -828 -860 MBr -517 -674 -715 -754 MI -360 -535 -567 -602
-
Richardson Co. acquires machinery by paying $10,000 cash. Richardson purchased a similar machine last month for $13,500. At what cost should the new equipment be recorded?
-
Companies that invest heavily in eco-friendly initiatives, such as transitioning to renewable energy sources or implementing carbon offset programs, may initially face increased operational costs....
-
Answer each question individually please. 14-13 What are the advantages and drawbacks of universities using social media to communicate with various stakeholdersstudents, potential students, alumni,...
-
act as a consultant hired by the operations director of the Barry Computer Company provide a financial analysis and comparison to the industry. You will conduct a financial ratio analysis to gain a...
-
Building a sense of community is not just a moral thing to do, but also a pragmatic one. In today's competitive and ever-evolving business environment, the organizations that can attract the most...
-
Watch https://youtu.be/U3MtvvNjUR4 What do you think of Dr. Saint's ideas about barriers to change? What do you think about social learning? Could this tool be used to make real change? How can the...
-
Organizations are increasingly using groups and teams in the workplace (for good reason, as it turns out, based on research results), and managers need to know how to work with people in such...
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
Figure 26.68 shows Mssbauer spectra of a sample of ferredoxin from chloroplasts at 77 K. Interpret the data with regard to the oxidation states an spin states of the two Fe atoms and comment on the...
-
J.A. Botas et al. discuss the catalytic conversion of vegetable oils into hydrocarbons suitable for use as biofuels (Catal. Today, 2012, 195, 59). What are the most important features of catalysts...
-
Apart from direct O-atom transfer (Fig. 26.50), another mechanism proposed for Mo enzymes is indirect O-atom transfer, also known as coupled electron-proton transfer. In this mechanism, shown in Fig....
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


