Figure 21.44 shows the change in concentration of [MnO 4 ] with time during a reaction
Question:
Figure 21.44 shows the change in concentration of [MnO4]− with time during a reaction with acidified oxalate ions.
(a) Suggest a method of monitoring the reaction.
(b) Explain the shape of the curve.
Figure 21.44
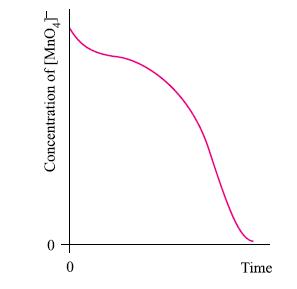
Transcribed Image Text:
Time Concentration of [MnO4]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The change in concentration of MnO4 with time during the reaction with acidified oxalate ions can be monitored using a spectrophotometer Spectrophot...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Did Jim and Laura Buy a Car? Jim and Laura Buyer visit the local car dealership because they are interested in buying a new car. The car they currently have is aging and is starting to have...
-
The graph shows the change in temperature as heat is supplied to a certain mass of ice initially at 80.0°C. What is the mass of the ice? 20 E-60 10 15 Heat (
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Go out onto the Web and compare three shopping bots for a product you are interested in (e.g., www.mysimon.com, www.bottomdollar.com, www.shopzilla.com, www.shopping.com, or www.pricegrabber.com)....
-
Here is a CPM network with activity times in weeks: a. Determine the critical path. b. How many weeks will the project take to complete? c. Suppose F could be shortened by two weeks and B by one...
-
Toyota Motor Manufacturing, USA, INC., Kazuhiro Mishina and Kazunori Takeda, Harvard Business School (1995): What are the issues with the seat installation?
-
What is the so-called Norwalk Agreement? LO4 a. An agreement between the FASB and SEC to allow foreign companies to use IFRSs in their fil ing of financial statements with the SEC. b. An agreement...
-
The data set agsrs.dat also contains information on the number of farms in 1987 for the SRS of n = 300 counties from the population of the N = 3078 counties in the United States (see Example 2.5). In...
-
Please help by filling in the blanks. Thank you! Pickle Motorcycles, Inc. (PMI), manufactures three motorcycle models: a cruising bike (Route 66), a street bike (Main Street), and a starter model...
-
(a) Explain the origins of MLCT and LMCT absorptions in the electronic spectra of d-block metal complexes. Give examples to illustrate your answer. (b) Explain what information can be obtained from a...
-
How would you attempt to (a) Estimate the crystal field stabilization energy of FeF 2 , (b) Determine the overall stability constant of [Co(NH 3 ) 6 ] 3+ in aqueous solution given that the overall...
-
1. What is design thinking? How does it differ from traditional models of decision making? 2. In your opinion, should most organizations adopt a design-thinking perspective? Why or why not? 3....
-
1.A woman eats 65g of protein per day. She weighs 156 lbs. How much protein is she getting per kg of body weight? Explain briefly both question 2.152 lbs = ________________kg. [2.2 lbs = 1 kg] explain
-
The concrete slab shown in Figure is 7m x 5m. The slab is not supported along one of the 7m long edges (free edge). The other three edges are supported and continuous over the supports, and therefore...
-
1. If possible, find 4-B -[{3}][{3}] A=
-
Georgi owns 50% of Forbes, Inc., an S corporation. At the beginning of the current tax year, Georgi had zero basis and an unused net business loss carryover of $10,000. During the tax year, she...
-
luation, of Fundamental Managerial Accounting Concepts. Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the...
-
Augusta Inc. acquired 100% of the outstanding common stock of Dear Corporation in a business combination. Immediately before the business combination, the two businesses had the following balance...
-
What mass of H2 will be produced when 122 g of Zn are reacted? Zn(s) + 2HCl(aq) ( ZnCl2(aq) + H2(g)
-
Identify all products expected for each of the following reactions. Take stereochemistry into account, and draw expected stereoisomer(s), if any: (a) (b) (c) (d) NBS hv
-
Ambien TM is a sedative used in the treatment of insomnia. It was discovered in 1982 and brought to market in 1992 (it takes a long time for new drugs to undergo the extensive testing required to...
-
The following compound is known to be chiral. Draw its enantiomer, and explain the source of chirality. CH
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


