Given the following standard potentials in basic solution and assuming that a reversible reaction can be established
Question:
Given the following standard potentials in basic solution
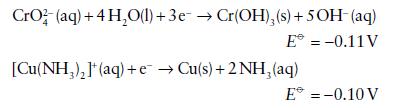
and assuming that a reversible reaction can be established on a suitable catalyst, calculate E⦵, ΔrG⦵, and K for the reductions of
(a) CrO42−
(b) [Cu(NH3)2]+ in basic solution. Comment on why ΔrG⦵ and K are so different between the two cases despite the values of E⦵ being so similar.
Transcribed Image Text:
CrO2 (aq) + 4H₂O(l) + 3e → Cr(OH), (s) + 5OH- (aq) E = -0.11 V [Cu(NH₂)₂](aq) + e→Cu(s) + 2NH₂ (aq) E* = -0.10 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
To calculate E standard cell potential rG standard Gibbs free energy change and K equilibrium constant for the reductions of CrO42 and CuNH32 in basic ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Given the following standard hours of planned and actual inputs and outputs at a work centre, determine the WIP for each time period. The beginning WIP is seven hours of work. Period 2 Input Planned...
-
Given the following standard hours of planned and actual inputs and outputs at a work centre, determine the WIP for each period. The beginning WIP is 12 hours of work. eri 3 24 20 Planned 24 25 Pln...
-
Jarvis Company produces a product that has a selling price of $20.00 and a variable cost of $15.00 per unit. The company's fixed costs are $50,000. What is the break-even point measured in sales...
-
Multiple Choice Questions 1. The intersection between demand for U.S. dollars and the supply of U.S. dollars is known as the a. Inflation rate. b. Exchange rate. c. Price. d. quantity. 2. An...
-
Choose one of the massively multiplayer online role-playing games (MMORPGs). Examples include World of Warcraft, Eve Online, EverQuest, Lineage, and Star Wars Galaxies. What leadership skills are...
-
Are we getting smarter? When the Stanford-Binet IQ test came into use in 1932, it was adjusted so that scores for each age group of children followed roughly the Normal distribution with mean 100 and...
-
Complete the following statements by filling in the blanks. (a) In a period in which a taxable temporary difference reverses, the reversal will cause taxable income to be ___________ (less...
-
a clearer version: how to calculate the following questions, Q1-5? clearer numbers 1 calculation till You are the revenue manager for the Hotel SHITM (300). The below information was the booking...
-
Characterize the condition of acidity or basicity that would most favour the following transformations in aqueous solution: (a) Mn 2+ MnO 4 , (b) ClO 4 ClO 3 , (c) H 2 O 2 O 2 , (d) I 2 2I .
-
Consult the Ellingham diagram in Fig. 6.16 and determine if there are any conditions under which aluminium might be expected to reduce MgO. Comment on these conditions. Figure 6.16. A,G*/ (kJ md-)...
-
A pistoncylinder device initially contains 0.8 m3 of saturated water vapor at 250 kPa. At this state, the piston is resting on a set of stops, and the mass of the piston is such that a pressure of...
-
James A. and Ella R. Polk, ages 70 and 65, respectively, are retired physicians who live at 3319 Taylorcrest Street, Houston, Texas 77079. Their three adult children (Benjamin Polk, Michael Polk, and...
-
I need help solving the following question: - Thank you in advance. On January 1, Year 6, HD Lid., a building supply company, JC Lid., a construction company, and Mr. Saeid, a private investor,...
-
Let X 1 , , X n X 1 , , X n be a random sample from a normal distribution with mean and variance 1. Find the uniformly minimum variance unbiased estimator of 2 2 .2 answers
-
The ledger of Duggan Rental Agency on March 31 of the current year includes the following selected accounts before adjusting entries have been prepared. Debit Credit Prepaid Insurance $3,600 Supplies...
-
1.Using the Excel file Sales transaction find the following15 Marks a.Identify the levels of measurement for each variables b.Construct a cross tabulation to find the number of transactions by...
-
Refer to the International Conference on Social Robotics (Vol. 6414, 2010) study of the trend in the design of social robots, Exercises 3.10 and 3.37. Recall that in a random sample of 106 social...
-
Let (X. A. p) be a measure space. Show that for any A,B A, we have the equality: (AUB)+(An B) = (A) + (B).
-
(a) What is the function of cytochrome c oxidase? (b) Describe the four active metal-containing sites in cytochrome c oxidase and the proposed way in which they work together to fulfil the role of...
-
(a) Outline the similarities and differences between the haem units in deoxymyoglobin and cytochrome c. (b) What function does cytochrome c perform in mammals?
-
Comment on the similarities and differences between a [2Fe2S] ferredoxin and Rieske protein, in terms of both structure and function.
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


