How do you account for the fact that, although potassium is placed after argon in the periodic
Question:
How do you account for the fact that, although potassium is placed after argon in the periodic table, it has a lower relative atomic mass?
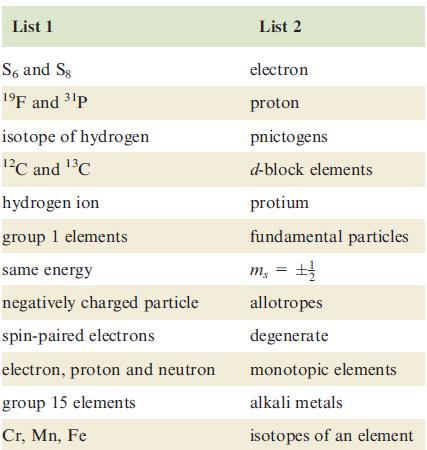
Transcribed Image Text:
List 1 S6 and Sg 1⁹F and ³¹p isotope of hydrogen ¹²℃ and ¹³℃ hydrogen ion group 1 elements same energy negatively charged particle spin-paired electrons electron, proton and neutron group 15 elements Cr, Mn, Fe List 2 electron proton pnictogens d-block elements protium fundamental particles m₁ = ±1/ allotropes degenerate monotopic elements alkali metals isotopes of an element
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
The apparent discrepancy between the placement of potassium and argon in the periodic table and thei...View the full answer

Answered By

Kratika Agrawal
I have already worked as an subject experts.
Hello! After reviewing your assignment criteria, I am certain than I can provide the greatest for you by the deadline. This assignment is the area of my specialization kindly considers my proposal and get a quality paper. Thanks!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
IST 8100 Supplemental Cases Inside Microsoft During last fall's United Way campaign at Microsoft, two vice-presidents made a wager on whose division would generate the most contributions. The loser,...
-
In what ways does the clinical approach to selection differ from the statistical approach? How do you account for the fact that one approach is superior to the other?
-
A uniform marble rolls down a symmetric bowl, starting from rest at the top of the left side. The top of each side is a distance h above the bottom of the bowl. The left half of the bowl is rough...
-
5 Melbourne Corporation has traditionally made a subcomponent of its major product. Annual production of 30,000 subcomponents results in the following costs: Direct materials Direct labor Variable...
-
Accounting has its own vocabulary and basic relationships. Match the accounting terms at the left with the corresponding phrase at the right. ____ 1. Posting ____ 2. Normal balance ____ 3. Payable...
-
Solve: a. x 2 25 0 b. x 2 + 7x + 10 0 c. x 2 + 6x 7 > 0 d. 14x 2 + 17x 6 0 e. 6x 2 23x + 20 < 0 f. 4 7x 2x 2 < 0
-
3. The proprietary fund statement of cash flows includes all of the following sections except: a Cash flows from operating activities b Cash flows from investing activities c Cash flows from capital...
-
Using the information in P4- 6 and P4- 7, perform the following steps for Tides Tea Company: In P4- 6 Tides Tea Company began operations on January 1, 2015. During the first year of business, the...
-
8 Strategic equity investments with significant influence are measured using: ut of Select one: a. All of the available choices. b. Equity Method c. Fair Value through Net Income Method d....
-
For a neutral atom, X, arrange the following atomic orbitals in an approximate order of their relative energies (not all orbitals are listed): 2s, 3s, 6s, 4p, 3p, 3d, 6p, 1s.
-
Write down the six sets of quantum numbers that describe the electrons in a degenerate set of 5p atomic orbitals. Which pairs of sets of quantum numbers refer to spin-paired electrons?
-
Which of the following is most likely to be unique to the audit work of certified accountants as compared to work performed by practitioners of other professions? (A) Due professional care. (B)...
-
How much Group Revenue do you currently have booked for September 2024? (format $, no decimals; e.g, $5,000) How much additional Group Revenue do you need to book to hit your budget for September...
-
What strategies do businesses have in place that promote equal opportunity within the organization? Do most businesses offer career development and training to its employees? If so, why? How do...
-
How do you do the step method in cost accounting? I need help understanding the difference between the step method and reciprocal method.
-
1- How do long-term care changing the health care system in the 21st century? What ethical issues do you see playing a role in this situation? 2- Why do long-term health care and palliative care...
-
How do the processes and layouts of Harley Davidson enable it to deliver goods and services to its customer? (How do they do what they do with the resources they have?)
-
A researcher wants to determine a model that can be used to predict the 28-day strength of a concrete mixture. The following data represent the 28-day and 7-day strength (in pounds per square inch)...
-
(a) Given a mean free path = 0.4 nm and a mean speed vav = 1.17 105 m/s for the current flow in copper at a temperature of 300 K, calculate the classical value for the resistivity of copper. (b)...
-
Explain why compounds of beryllium are mainly covalent whereas those of the other Group 2 elements are predominantly ionic.
-
Marble and limestone buildings are eroded by contact with acid rain. Define the term acid rain and discuss the origins of the acidity. Describe the processes by which the marble and limestone are...
-
Explain how the nature of the alkyl group affects the structure of lithium alkyls.
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


