In aqueous solution, boric acid behaves as a weak acid (pK a = 9:1) and the following
Question:
In aqueous solution, boric acid behaves as a weak acid (pKa = 9:1) and the following equilibrium is established:
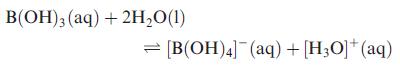
(a) Draw the structures of B(OH)3 and [B(OH)4]‾.
(b) How would you classify the acidic behaviour of B(OH)3?
(c) The formula of boric acid may also be written as H3BO3; compare the acidic behaviour of this acid with that of H3PO3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





