In each redox reaction in problem 8.3, confirm that the net increases and decreases in oxidation states
Question:
In each redox reaction in problem 8.3, confirm that the net increases and decreases in oxidation states balance each other.
Data from Problem 8.3
Which of the following reactions are redox reactions? In those that are, identify the oxidation and reduction processes.
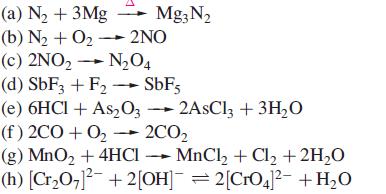
Transcribed Image Text:
(a) N₂ + 3Mg Mg3N₂ (b) N₂ + O₂- 2NO (c) 2NO₂ → N₂04 (d) SbF3 + F₂ → SbF5 (e) 6HCl + As2O32AsCl3 + 3H₂O (f) 2CO+O₂ → 2CO₂ (g) MnO₂ + 4HCI MnCl₂ + Cl₂ + 2H₂O (h) [Cr₂O7]²¯ + 2[OH]¯ = 2[CrO4]²¯ +H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
To determine whether each reaction is a redox reaction and confirm that the net increases and decreases in oxidation states balance each other we need to identify the oxidation and reduction processes ...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The commercial production of nitric acid involves the following chemical reactions: (a) Which of these reactions are redox reactions? (b) In each redox reaction identify the element undergoing...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Montage Pty Limited (Montage) is a resident private company and is not a base rate entity. Which of the following transactions would result in a debit entry to Montage's franking account? Payment of...
-
Using Peng Atlas Map 3.1, in your option would it be easier or more difficult to engage in competitive actions in those countries that are at the bottom?
-
Pierce Dussault, a lawyer, accepts a legal engagement in March, does the work in April, and is paid in May. If Dussaults law firm prepares monthly financial statements, when should it recognize...
-
Of the four growth strategies described in the chapter, which is the riskiest? Which is the easiest to implement? Why?
-
On May 21, 1927, Charles Lindbergh landed at Le Bourget Field in Paris, completing his famous transatlantic solo flight. The preparation period prior to his flight was quite hectic and time was...
-
Ford has two divisions: Xi and Sigma. Data from the most recent month appear below: Xi Sigma Sales $ 311,000 $ 346,000 Variable expenses $ 65,310 $ 169,540 Traceable fixed expenses $ 176,000 $...
-
(a) Calculate E Ag + /Ag for a half-cell in which the concentration of silver(I) ions is 0.1moldm 3 (T = 298 K). (b) Are silver(I) ions more or less easily reduced by zinc in this solution than under...
-
Consider the half-reaction: If the ratio of concentrations of [MnO 4 ] : Mn 2+ is 100:1, determine E at pH values of (a) 0.5; (b) 2.0; (c) 3.5 (T = 298 K). Over this pH range, how does the ability...
-
Interview individuals who represent the three stages of the financial life cycle about their credit card usage. How many cards do they have? What kind or class of cards (rebate, premium, affinity,...
-
In the introduction to "The Five Sexes," Anne Fausto-Sterling writes that she had to "invent conventions - s/he and his/her - to denote someone who is clearly neither male nor female or who is...
-
Select a product described as one of the "Biggest Product Flops" of 2019 that you will bring back to the market. To, you will need to engage in some research to understand why the product failed to...
-
Breaking the Bank Case Questions (video found at: http://www.pbs.org/wgbh/pages/frontline/breakingthebank/view/?utm_campaign=viewpage &utm_medium=grid&utm_source=grid) 1) To what extent were the...
-
Please answer in full and write legibly. Suppose Alice has taken 7 classes college, and her current GPA is 3.48 (assume for simplicity that all courses carry the same number of credits). Answer the...
-
F. Explain how to overcome two potential biases (e.g., prejudice, discrimination) using culturally competent strategies that will help improve stakeholder communication. G. Explain how to mitigate...
-
The National Highway Traffic Safety Administration publishes reports about motorcycle fatalities and helmet use. The distribution shows the proportion of fatalities by location of injury for...
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
Describe the inorganic chemistry involved in the operation of a lambda sensor (an exhaust-gas oxygen sensor) in a vehicle engine.
-
Identify the likely products of the reactions (a) Li,CO,+CoO_800C,0_ (b) 2 Sr(OH) +WO+MnO 900C,0,
-
Outline how you could prepare samples of (a) MgCr 2 O 4 , (b) LaFeO 3 , (c) Ta 3 N 5 , (d) LiMgH 3 , (e) KCuF 3 , (f) The zeolite A analogue with Ga replacing Al, Na 12 [Si 12 Ga 12 O 48 ]nH 2 O.
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


