In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The 19 F
Question:
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed.
The 19F NMR spectrum of the octahedral ion [PF5Me]‾ shows two signals (δ – 45.8 and –57.6 ppm). Why are two signals observed? From these signals, three coupling constants can be measured: JPF = 829 Hz, JPF = 680 Hz and JFF = 35 Hz. Explain the origins of these coupling constants.
Table 4.3
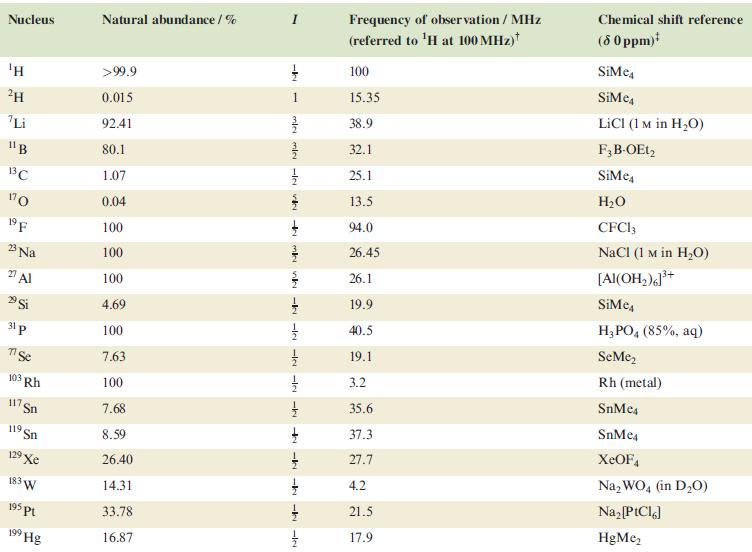
Transcribed Image Text:
Nucleus ΤΗ ²H Li 11B 13C 170 19 F 23 Na 2 Al 29 Si 31 p 7 Se 103 Rh 117. Sn 119 Sn 19 Xe 183 W 195 pt 199 Hg Natural abundance/% >99.9 0.015 92.41 80.1 1.07 0.04 100 100 100 4.69 100 7.63 100 7.68 8.59 26.40 14.31 33.78 16.87 miele v + mk nh -k -le -le-le - -ki -k -k -IN Frequency of observation / MHz (referred to ¹H at 100 MHz) 100 15.35 38.9 32.1 25.1 13.5 94.0 26.45 26.1 19.9 40.5 19.1 3.2 35.6 37.3 27.7 4.2 21.5 17.9 Chemical shift reference (8 0 ppm)* SiMe SiMe4 LICI (1 M in H₂O) F3B-OEt2 SiMe H₂O CFC13 NaCl (1 M in H₂O) [Al(OH₂)]³+ SiMc4 H3PO4 (85%, aq) SeMe₂ Rh (metal) SnMc4 SnMe4 XeOF4 Na₂WO4 (in D₂O) Na₂ [PtCl6] HgMe₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
The presence of two signals in the 19F NMR spectrum of the octahedral ion PF5Me is due to the inequivalent fluorine nuclei in the molecule In octahedr...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
19F is the only isotope of fluorine that occurs naturally, and it has a nuclear spin of + (a) Into how many peaks will the proton signal in the 1H NMR spectrum of methyl fluoride be split? (b) Into...
-
19F is the only isotope of fluorine that occurs naturally, and it has a nuclear spin of 1/2 (a) Into how many peaks will the proton signal in the 1H NMR spectrum of methyl fluoride be split? (b) Into...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. (a) In the 1 H NMR spectrum of compound 4.21, there is a triplet at 3.60 ppm (J 10.4 Hz). Assign the signal and...
-
The following are selected 2023 transactions of Ayayai Corporation. Sept. 1 Oct. 1 1 Purchased inventory from Indigo Ltd. on account for $47,200. Ayayai uses a periodic inventory system. Issued a...
-
Place the corresponding letter of the definition next to the term. _____ 1. Cost principle _____ 2. Business entity principle _____ 3. Generally accepted accounting principles _____ 4. Reliability...
-
Suppose you train a classifier and test it on a held-out validation set. It gets 30% classification accuracy on the training set and 30% classification accuracy on the validation set. a. From what...
-
1 Was the interview fair and unbiased? What is your overall assessment of the way in which Bill conducted the interview? How could his conduct of the interview have been improved?
-
Comprehensive Income Armstrong Corporation reported the following for 2010: net sales $1,200,000 cost of goods sold $720,000: selling and administrative expenses $320,000: and an unrealized holding...
-
John Fleming, chief administrator for Valley View Hospital, is concerned about the costs for tests in the hospital's lab. Charges for lab tests are consistently higher at Valley View than at other...
-
(a) If Na has the ground state electronic configuration of [Ne]3s 1 , why is NaCl EPR silent? (b) Sketch an EPR spectrum for an isotropic system in which an electron interacts with a 14 N (I = 1)...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The 31 P{ 1 H} NMR spectrum of a CDCl 3 solution of the square planar rhodium(I) complex 4.22 exhibits a doublet of...
-
As the manager of a Mama Bodhis restaurant, you must deal with a variety of business transactions. Give an example of a transaction that has each of the following effects on the accounting equation:...
-
How do multi-track diplomacy frameworks, integrating official, unofficial, and grassroots efforts at different levels of society, enhance the effectiveness and inclusivity of conflict resolution...
-
As explained by Welch, what should managers do to determine what their own organizations have been up to ?
-
As an administrator how do you demonstrate below situation with suitable examples. 1 Completes tasks to a high standard 2 Demonstrates the necessary level of expertise required to complete tasks and...
-
What influences do the pharmaceutical companies have on psychiatry? What acronym can guide you in formulating a treatment plan (hint: Your instructor emphasizes this when creating a treatment plan,...
-
How do you write a board paper from an article? for example how would y a board paper from the article below look like? Aritcle...
-
Given the following ANOVA output, answer the questions that follow. (a) Is there evidence of an interaction effect? Why or why not? (b) Based on the P-value, is there evidence of a difference in the...
-
Q:1 Take any product or service offered in Pakistan and apply all determinents of customer Perceived value ?
-
The potential of organofluoro compounds in materials chemistry is discussed in a paper by R. Berger and co-workers (Chem. Soc. Rev., 2011, 40, 3496). One group of compounds discussed is the...
-
Sketch a chloralkali cell. Show the half-cell reactions and indicate the direction of diffusion of the ions. Give the chemical equation for the unwanted reaction that would occur if OH migrated...
-
In their paper Recent discoveries of polyhalogen anions from bromine to fluorine (Z. Anorg. Allg. Chem., 2014, 640, 7, 1281), H. Haller and S. Riedel described the synthesis and structure elucidation...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


