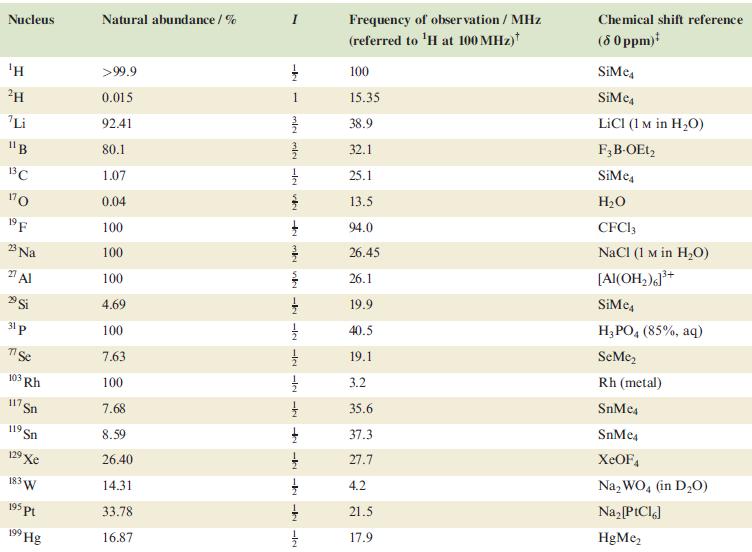
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The structure of
Question:
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed.
The structure of [P5Br2]+ is shown in diagram 4.17. Account for the fact that the 31P NMR spectrum of this cation at 203K consists of a doublet of triplets (J 321 Hz, 149 Hz), a triplet of triplets (J 321 Hz, 26 Hz) and a triplet of doublets (J 149 Hz, 26 Hz).
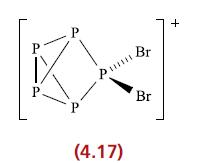
Table 4.3
Transcribed Image Text:
P P P P pun P (4.17) Br Br +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To interpret the NMR spectrum of P5Br2 we first need to understand its molecular structure and the potential interactions between the phosphorus nucle...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Rationalize the fact that the 13 C NMR spectrum of CF 3 CO 2 H consists of two binomial quartets with coupling...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. (a) Predict the structure of SF 4 using the VSEPR model. (b) Account for the fact that at 298K and in solution the...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. NaBH 4 contains the tetrahedral [BH4] ion. Although NaBH 4 hydrolyses slowly in water, it is possible to obtain a...
-
Hi, I need help with this accounting problem, thanks inadvance.l [The following information applies to the questions displayed below.] Delph Company uses a job-order costing system and has two...
-
Marybeth Jones is the controller at Patterson Supply Company, a publicly traded distributor of floral supplies. It is the end of the third quarter and Marybeth is working under a deadline to get the...
-
The points A(0, 0), B(0.5, 0.75), C(0.8, 1.44), D(0.95, 1.8525), E(0.99, 1.9701) and F(1, 2) lie on the curve y = f(x). a. Copy and complete the table to show the gradients of the chords CF, DF and...
-
Cost of indirect material is apportioned to various departments. State whether the following statements are true or false:
-
Transactions related to revenue and cash receipts completed by Sycamore Inc. during the month of March 20Y8 are as follows: Mar. 2. Issued Invoice No. 512 to Santorini Co., $905. 4. Received cash...
-
the set of assumptions underlying the firm's financial plan and the resulting projected financial statements are accordingly often referred to as which of the following? 1. Base case projections. 2....
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Is it correct to interpret the phrase static solution structure as meaning necessarily rigid? Use the following...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Outline the mechanism of Berry pseudo-rotation, giving two examples of molecules that undergo this process. Table...
-
In the example given by Ms. Plain, what was the spread for the GHT stock just prior to execution? a. $0.06 b. $0.02 c. $0.04 George White, CFA, and Elizabeth Plain, CFA, manage an account for Briggs...
-
Required information Use the following information for the Exercises below. (Algo) [The following information applies to the questions displayed below.] Ramirez Company installs a computerized...
-
Reproduced below from Farthington Supply s accounting records is the accounts receivable subledger along with selected general ledger accounts. General Ledger Accounts Receivable Dec. 3 1 / 2 2...
-
James A. and Ella R. Polk, ages 70 and 65, respectively, are retired physicians who live at 3319 Taylorcrest Street, Houston, Texas 77079. Their three adult children (Benjamin Polk, Michael Polk, and...
-
Required information [The following information applies to the questions displayed below.] Shauna Coleman is single. She is employed as an architectural designer for Streamline Design (SD). Shauna...
-
The following are the ratings of men by women in an experiment involving speed dating. Use the given data to construct a boxplot and identify the 5-number summary. 3.0 3.5 4.0 4.5 5.5 5.5 6.5 6.5 6.5...
-
How does the completely randomized design differ from a randomized complete block design?
-
Consider the activities undertaken by a medical clinic in your area. Required 1. Do you consider a job order cost accounting system appropriate for the clinic? 2. Identify as many factors as possible...
-
Explain how you could use 31 P-NMR to distinguish between PF 3 and POF 3 .
-
(a) Use standard potentials to calculate the standard potential of the disproportionation of H 2 O 2 in acid solution. (b) Is Cr 2+ a likely catalyst for the disproportionation of H 2 O 2 ? (c) Given...
-
Give balanced chemical equations for the reactions of the following reagents with PCl 5 and indicate the structures of the products: (a) Water (1:1), (b) Water in excess, (c) AlCl 3 , (d) NH 4 Cl.
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


