In the description of the bonding of B 2 H 6 , we draw the conclusion that
Question:
In the description of the bonding of B2H6, we draw the conclusion that the two bonding MOs in Fig. 5.33 have B—H bonding character delocalized over the four bridge atoms.
(a) What other character do these MOs possess?
(b) Does your answer to (a) alter the conclusion that this approximate MO description is consistent with the valence bond idea of there being two 3c-2e bridge bonds?
Figure 5.33
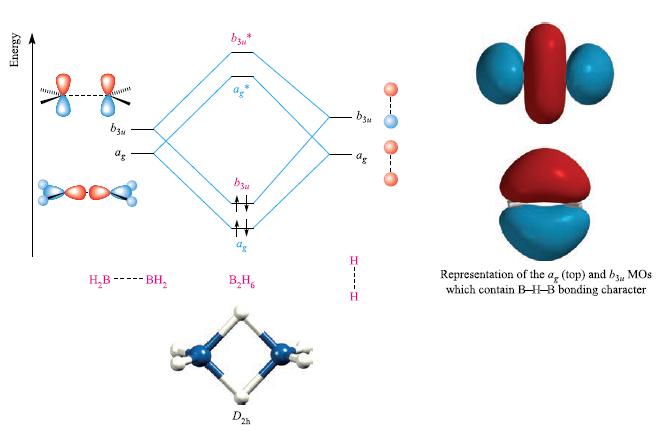
Transcribed Image Text:
Energy && OHARRE by ag H₂B ----- BH₂ b3 08 bu #: # B.H D b3u Н as H----H ✿ Representation of the a, (top) and b3, MOS which contain B-H-B bonding character
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a The two bonding molecular orbitals MOs in Fig 533 which have BH bonding character delocalized over ...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In our development of consumer theory, we made a big point about the fact that neoclassical economics does not put much stock in the idea of cardinally measuring utility (in terms of units of...
-
There are many equity or fairness based arguments for government engagement in anti-poverty programs and for general government redistribution programs. But is there an efficiency case to be made...
-
We have illustrated in several settings the role of actuarially fair insurance contracts (b, p) (where b is the insurance benefit in the bad state and p is the insurance premium that has to be paid...
-
All organizations incur non value added costs. Eliminating or reducing non value added costs is the responsibility of managers. Using the Activity Based Management approach and focusing on the...
-
What are examples of how formal institutions affect marketing and supply chain management-i.e., examples of government-imposed rules of the game?
-
Given below is some is a comparison of financial performance data of a project when flexibility is incorporated (I.e. flexible project) in comparison to when it is not. (i.e. inflexible project) The...
-
Births to unwed mothers. Here are data on births to unwed mothers as a percentage of births in the United States, from the Statistical Abstract. These data show a clear increasing trend over time....
-
A local partnership is considering possible liquidation because one of the partners (Bell) is insolvent. Capital balances at the current time are as follows. Profits and losses are divided on a...
-
7- A college saving fund is to be established by a single payment so that at the end of 15 years, there will be $50,000 in the fund. If the fund earns interest at the rate of 8% compounded annually,...
-
What is meant by a ligand group orbital?
-
The hydrido complex [FeH 6 ] 4 has Oh symmetry. The bonding in [FeH 6 ] 4 can be described in terms of the interactions between the atomic orbitals of Fe and the LGOs of the H 6 -fragment. (a)...
-
The frontal lobes become fully developed ________. a. At birth b. At the beginning of adolescence c. At the end of adolescence d. By 25 years old
-
RQ3: How might finance affect the formulation and application of corporate strategies?
-
How does this scene relate to a person who is?
-
How does Max learn to apologize?
-
Why are some on the spectrum bullied, and how must a parent feel?
-
Question 1: Response to Theresa Johnson?
-
Explain the differences between the chi-square test for independence and the chi-square test for homogeneity. What are the similarities?
-
What is an access control list?
-
Figure 26.68 shows Mssbauer spectra of a sample of ferredoxin from chloroplasts at 77 K. Interpret the data with regard to the oxidation states an spin states of the two Fe atoms and comment on the...
-
J.A. Botas et al. discuss the catalytic conversion of vegetable oils into hydrocarbons suitable for use as biofuels (Catal. Today, 2012, 195, 59). What are the most important features of catalysts...
-
Apart from direct O-atom transfer (Fig. 26.50), another mechanism proposed for Mo enzymes is indirect O-atom transfer, also known as coupled electron-proton transfer. In this mechanism, shown in Fig....
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


