Predict the shapes of the following molecules or ions: (a) CICN; (b) OCS; (c) [SiH3]; (d) [SnCl,];
Question:
Predict the shapes of the following molecules or ions:
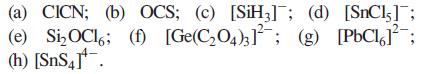
Transcribed Image Text:
(a) CICN; (b) OCS; (c) [SiH3]; (d) [SnCl,]; (e) Si₂OCl; (f) [Ge(C₂O4)3]; (g) [PbCl²; (h) [SnS4].
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
To predict the shapes of molecules or ions we need to consider their molecular geometries based on the number of bonding pairs and lone pairs of electrons around the central atom The shapes are determ...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a) What is the bond order of F?OF based on the Lewis structureof the molecule? b) What is the bond order of O2 based on theLewis structure of the molecule? c) What is the bond order of CO based on...
-
a. Predict the shapes of the following molecules i. Tetrachloromethane, CCl 4 ii. Beryllium chloride, BeCl 2 iii. Phosphorus(III) chloride. b. Draw dot-and-cross diagrams for the following molecules...
-
The following molecules and ions are grouped by similar structures. Classify each as aromatic, antiaromatic, or nonaromatic. For the aromatic and antiaromatic species, give the number of pi electrons...
-
1. If an industry is formed by six companies. Four companies have sales of $ 10 each, and two companies have sales of $ 5 each. a. What is the concentration ratio of four companies for this industry?...
-
The Internet is a good place to get information that is useful to you in your study of accounting. For example, you can find information about accounting firms, standard setters, and regulators....
-
Using the internet and your textbook, research the following: Functionality and purpose of each layer of the OSI Model Details about what and how communication occurs up and down the OSI stack Any...
-
If necessary, start MOA and open the service based sample company, Fabrikam, Inc. Restore the Chapter 7.sbb backup file. This backup was made on page 213. Open Fabrikam, Inc. Answers the questions...
-
Trekster Corrector guarantees its snowmobiles for three years. Company experience indicates that warranty costs will be approximately 4% of sales. Assume that the Trekster dealer in Colorado Springs...
-
Required information The following information applies to the questions displayed below.) Tree Seedlings has the following current-year purchases and sales for its only product. Units sold at Units...
-
In 1 H NMR spectra in which the solvent is acetonitrile-d 3 , labelled to an extent of 99.6%, a multiplet is observed at 1.94 ppm. How does this multiplet arise, and what is its appearance? [D, I =...
-
Comment on each of the following observations. (a) The carbides Mg 2 C 3 and CaC 2 liberate propyne and ethyne respectively when treated with water, reaction between ThC 2 and water produces mixtures...
-
The halo effect can come into play for lots of reasons: a pleasant personality, a shared work history (i.e., you both worked for the same company in the past) or a shared network of business...
-
Think back to a time you experienced a communication breakdown in a personal or social setting (something you're comfortable discussing with the class in a public forum). 1. Did you figure out why...
-
Imagine you are visiting your aunt, who is a patient in a hospital in a nearby city. While you are sitting at her bedside, you hear a lot of noise at the nurses' station, as if they are having a...
-
Using Houseplan #5 on page 4 of the Measurement supplement(below), determine the cost of pouring the 9 inch thick concreteslab for this home, assuming that the porch will also be on thefoundation....
-
Recall from lecture that Flip-Flap Railway is an old roller coaster that was built in a circle. It has a diameter of 25 ft and riders entered the ride at a speed of 45 mph. At the top of the loop,...
-
Small Fry Design, founded in 1997, is a toy and accessories company that designs and imports products for children. The company's line of merchandise includes teddy bears, musical toys, rattles and...
-
What competitive advantage in the workplace do you possess that provides value for current and future employers? How can you fortify this advantage in support of your career growth and movement?
-
Reichenbach Co., organized in 2018, has set up a single account for all intangible assets. The following summary discloses the debit entries that have been recorded during 2018 and 2019. Instructions...
-
In an article on the detection of Zn(II) that is released from neuronal tissue (brain, nerves) following trauma, E. Tomat and S.J. Lippard describe the development of special ligands that are highly...
-
Predict the properties of Na + , Ca 2+ , and Cl binding sites that would be important for providing selectivity in their respective transmembrane ion transporters.
-
Ultimately, the overall green credentials of any process can only be evaluated through life cycle analysis (LCA). Identify what components would be considered in the LCA of the synthesis of ammonia...
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


