Predict whether the equilibrium constants for the following reactions should be greater than 1 or less than
Question:
Predict whether the equilibrium constants for the following reactions should be greater than 1 or less than 1:
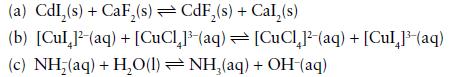
Transcribed Image Text:
(a) CdI₂(s) + CaF₂(s) CdF₂(s) + Cal,(s) (b) [Cul 1¹-(aq) + [CuCl₂]³(aq) (c) NH;(aq) +H,O(l)=NH,(aq) [CuCl²-(aq) + [Cul 1³ (aq) + OH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To predict whether the equilibrium constants Kc for the given reactions are greater than 1 or less t...View the full answer

Answered By

Deepankur Keserwani
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
(a) Based on the following energy profile, predict whether kf > or kf (b) Using Equation 15.5, predict whether the equilibrium constant for the process is greater than 1 or less than 1. 6r Reactants...
-
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the equation:
-
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the equation:
-
Governmental Funds Statement of Revenues Expenditures and Changes in Fund Balance. You have recently started working as the controller for a small county. The county is preparing its financial...
-
Use the standard price and cost data supplied in Problem 8-20B. Assume that Truebody actually produced and sold 432,000 units. The actual sales price and costs incurred follow. Actual price and...
-
Solve the IVP with y(0) = 0 using ode45. dy dt = e~! (4.10.1)
-
Give the accounting procedure for transport costing.
-
Calculate the profitability index for Problem 3. For Problem 4.
-
Go through the previous years results from the McDonalds website and understand if the Financial statement forecast is positive.
-
The f-block elements are found as M(III) lithophiles in silicate minerals. What does this indicate about their hardness?
-
Is the dissolution of silicates in HF a Lewis acidbase reaction, a Brnsted acidbase reaction, or both?
-
Identify the characteristics of universal life insurance.
-
how could playing in a sandbox help to the development of children? how could a garden help to the development of children? how could playground obstacle courses like a pebble bridge and monkey bars...
-
A store order bottles of shampoo throughout the year. Over time, the store has learned that the annual demand D for shampoo is constant, i.e., there is no variability. Currently, the store decides to...
-
Solve the Practice #2 == where L2 =02A = a, L404B = c, L = 0204 = d, y = /2 1) Find the velocity 3 when 82 = /2 and 6 = 0.4 rad/s 2) Find the acceleration 63 when = /2 and 62 = 0.4 rad/s 03. 03 Y B...
-
.0.5 0.5 For the above plot of the ellipsoid (22) 2- + +() + (-) = 1, find the parameters a, b and c. Note that a, b and c are positive integers between 1 and 6 inclusive. Use the mouse to rotate the...
-
The annual energy consumption of the University of Maryland is 100 million kWh. How much Uranium-235 is needed to produce this amount of energy in a nuclear power plant assuming 100% efficiency? (The...
-
The debate about centralization and decentralization is heating up again with the advent of Bring Your Own Device (B.Y.O.D.) and the increasing use of the Web. Why does the Internet make this debate...
-
Pedro Bourbone is the founder and owner of a highly successful small business and, over the past several years, has accumulated a significant amount of personal wealth. His portfolio of stocks and...
-
(a) Give a definition of lattice energy. Does your definition mean that the associated enthalpy of reaction will be positive or negative? (b) Use the BornLande equation to calculate a value for the...
-
(a) Draw a set of resonance structures for the hypothetical molecule PH 5 , ensuring that P obeys the octet rule in each structure. Assume a structure analogous to that of PF 5 . (b) To what point...
-
Refer to Table 6.2. (a) Write an equation for the process for which the standard enthalpy of atomization of cobalt is defined. (b) Suggest reasons for the trend in standard enthalpies of atomization...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


