Select the compound on each line with the named characteristic and state the reason for your choice.
Question:
Select the compound on each line with the named characteristic and state the reason for your choice.
(a) Strongest Lewis acid:
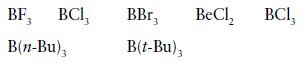
(b) More basic towards B(CH3)3
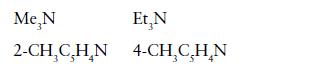
Transcribed Image Text:
BF, 3 BCI, B(n-Bu), BBr3 B(t-Bu) 3 BeCl₂ BC1₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To determine the strongest Lewis acid from the given compounds we need to understand what makes a species a strong Lewis acid A Lewis acid is a specie...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
For matrices A and B, find A 2B. A || 32 3 -1 4 0-2 2 B = 145 -1 -2 3
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
For each of the following situations, identify the inventory method that you would use or, given the use of a particular method, state the strategy that you would follow to accomplish your goal: a....
-
Marks 1. Find the limits, if they exist. If a limit does not exist, check whether the function approaches +00 x2 + 2x - 15 (5) (a) lim x-3 x2-4x +3 x2 - 4 (5) (b) lim x2 x4 - 16 Carol Ferland CF...
-
Strauss Company established a predetermined variable overhead cost rate at $10.50 per direct labor hour. The actual variable overhead cost rate was $9.60 per hour. The planned level of labor activity...
-
Design a parallel priority interrupt hardware for a system with eight interrupt sources.
-
Brain size and intelligence. For centuries people have associated intelligence with brain size. A recent study used magnetic resonance imaging to measure the brain size of several individuals. The IQ...
-
A metal sign for a car dealership is a thin, uniform right triangle with base length b and height h. The sign has mass M. (a) What is the moment of inertia of the sign for rotation about the side of...
-
business statistics is the subject please need answer of all parts in an hours. thanks in advance need it fast urgent pls just got 10 minutes left heading Heading a Question ! (10 marks) A company...
-
Draw the structures of chloric acid and chlorous acid and predict their pK a values using Paulings rules.
-
The reversible reaction of CO 2 gas with aqueous emulsions of long-chain alkyl amidine compounds has important practical applications. Describe the chemistry that is involved in this demonstration of...
-
This exercise should be used in conjunction with Exercises 1 and 3. Implement a Sphere class with the following features. Declare two instance variablescenter (whose type is Point) and radius (whose...
-
Reflect on your semester. How do you plan onmeasuringyour professionalgrowth in the future? What were the most challenging topics to you? What topics felt more intuitive/easy? How do you plan on...
-
Aside from shareholders, who do you believe is the second stakeholder in whose interests the company should be concerned? Justify your response What will you do to ensure the company's success...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Do you believe NIL promotes "love of the game," or does it make college sports more about money and business? What are the most significant positive and negative effects of NIL, in your opinion? What...
-
Even well-managed organizations do not always work as efficiently and effectively as management would like. At Hewlett-Packard (HP), billions of dollars of product are being shipped - from computers...
-
Discuss Architecting Dynamic random-access memory and describe the importance of DRAM in Silicon Photonic Technology, Pidram Channel Organization, Pidram Bank Organization and Assessment of the...
-
Write the statement to store the contents of the txtAge control in an Integer variable named intAge.
-
The values of pK a (1) and pK a (2) for chromic acid (H 2 CrO 4 ) are 0.74 and 6.49 respectively. (a) Determine values of K a for each dissociation step. (b) Write equations to represent the...
-
(a) Write equations to show how you expect compounds 7.24 to 7.28 to dissociate in aqueous solution. (b) Suggest how compound 7.29 will react with NaOH in aqueous solution. What salts would it be...
-
(a) Two-electron reduction of B 5 H 9 followed by protonation is a convenient route to B 5 H 11 . What structural change (and why) do you expect the B 5 cage to undergo during this reaction? (b)...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


