Suggest explanations for the following observations. (a) Ammonium fluoride forms solid solutions with ice. (b) The viscosity
Question:
Suggest explanations for the following observations.
(a) Ammonium fluoride forms solid solutions with ice.
(b) The viscosity decreases along the series of liquids H3PO4 > H2SO4 > HClO4.
(c) Formic (methanoic) acid has a Trouton constant of 60.7 JK−1 mol−1.
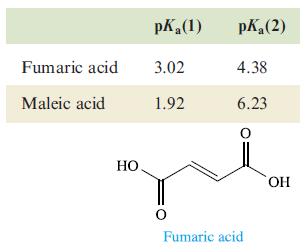
(d) pKa values for fumaric acid and its geometrical isomer maleic acid are:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




