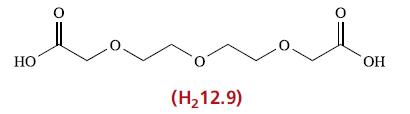
The reaction between Ca(OH) 2 and H 2 12.9 in aqueous solution leads to the formation of
Question:
The reaction between Ca(OH)2 and H212.9 in aqueous solution leads to the formation of the complex [Ca(OH2)2(12.9)]. This crystallizes as a centrosymmetric dimer in which each Ca2+ centre is 8-coordinate. This contains a central Ca2(μ-O)2 unit in which each bridging O-donor involves a carboxylate group; only one oxygen atom of each carboxylate group is coordinated. Propose a structure for the centrosymmetric dimer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





