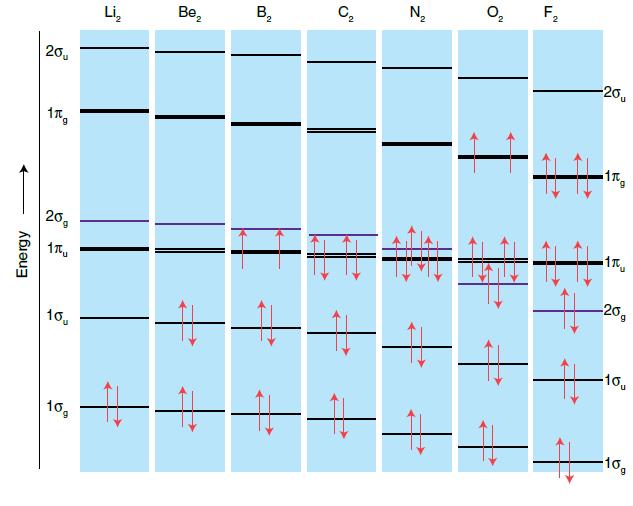
Use Fig. 2.17 to write the electron configurations of (a) Be 2 + , (b) B 2
Question:
Use Fig. 2.17 to write the electron configurations of
(a) Be2+,
(b) B2−,
(c) C2−,
(d) F2+ and sketch the form of the HOMO in each case.
Figure 2.17.
Transcribed Image Text:
Energy 20 1g 20 1. 100 10g Be2 B2 # # C2 N2 0% # F2 14414 → Z ← → -20 ・1 ・1 -20, -10, 18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
To write the electron configurations of Be2 B2 C2 and F2 using Fig 217 we need to identify the numbe...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Write the electron configurations for the following ions: (a) Fe2+, (b) Hg2+, (c) Mn2+, (d) Pt2+, (e) P3-.
-
Write an equation for the process that corresponds to the electron affinity of the ion. Also write the electron configurations of the species involved. What is the magnitude of the energy change in...
-
The electron configurations described in this chapter all refer to gaseous atoms in their ground states. An atom may absorb a quantum of energy and promote one of its electrons to a higher-energy...
-
Three partners. Ankamah, Kofi and David share profit and losses in the ratio 5: 3:2 for the year ending 31/12/2016. The profit generated from either business is $600,000. Partners' contributions are...
-
Keenan Co. is expected to maintain a constant 4.8 percent growth rate in its dividends indefinitely. If the company has a dividend yield of 6.9 percent, what is the required return on the companys...
-
Cycle Travel Tours showed the following trial balance information (in alphabetical order) for its first month just ended March 31, 2023: Required Use the information provided to complete an income...
-
8. Why is it much riskier to take a short position in a stock than a long position? What does that mean for the likelihood of overvaluation versus undervaluation of a companys share price?
-
Early in September 1983, it took 245 Japanese yen to equal $1. Nearly 25 years later, in May 2008, that exchange rate had fallen to 103.5 yen to $1. Assume that the price of a Japanese-manufactured...
-
Charlene can afford car payments of 185 a month for 48 months. If the interest rate is 5.65%, how much can she afford to borrow?
-
The common forms of nitrogen and phosphorus are N 2 (g) and P 4 (s), respectively. Account for the difference in terms of the single and multiple bond enthalpies.
-
Given that B(Si=O) = 640 kJ mol 1 , show that bond enthalpy considerations predict that siliconoxygen compounds are likely to contain networks of tetrahedra with SiO single bonds and not discrete...
-
A 1400 kg car moving at 5.3m/s is initially traveling north along the positive direction of a y axis. After completing a 90o right-hand turn rn 4.6 s, the inattentive operator drives into a tree,...
-
From Hoffman, what are the symptoms of autism and ADHD? From the Mayo Clinic, what are the causes and risk factors for autism and ADHD? What are treatment options for these disorders? Hofmann, S. G....
-
Brooks, a participant in the Zappa retirement plan, has requested a second plan loan. His vested account balance is $80,000. Brooks borrowed $27,000 eight months ago and still owes $18,000 on that...
-
Suppose that Angelina and Brad own the only two professional photography stores in town. Each must choose between a low price and a high price for senior photo packages. The annual economic profit...
-
based on the article How Chili's Is Prepping for Tough Times, Starting With the Fries by Heather Haddon. What is corporate social responsibility, and what is one way that Chili's can better pursue...
-
QUESTION 2 (20 marks) CLO 5 a. Explain what the following ratios indicate to a firm: (i) Acid Test Ratio (ii) Return on Capital Employed (ROCE) (iii) Debtors Collection Period (iv) Working Capital (2...
-
Who has the right to order that payment on a check be stopped?
-
Find the inverse, if it exists, for the matrix. -1
-
Refer to Table 6.2. (a) Write an equation for the process for which the standard enthalpy of atomization of cobalt is defined. (b) Suggest reasons for the trend in standard enthalpies of atomization...
-
(a) What hybridization scheme would be appropriate for the Si atom in SiH 4 ? (b) To which point group does SiH 4 belong? (c) Sketch a qualitative MO diagram for the formation of SiH 4 from Si and an...
-
By considering the structures of the following molecules, confirm that the point group assignments are correct: (a) BH 3 , D 3 h ; (b) NH 3 , C 3 v ; (c) B 2 H 6 , D 2 h .
-
This short exercise demonstrates the similarity and the difference between two ways to acquire plant assets. (Click the icon to view the cases.) Compare the balances in all the accounts after making...
-
Balance sheet and income statement data for two affiliated companies for the current year appear below: BALANCE SHEET As at December 31, Year 6 Albeniz Bach Cash $ 40,000 $ 21,000 Receivables 92,000...
-
please reference excel cells Caroll Manufacturing company manufactures a single product. During the past three weeks, Caroll's cost accountant observed that output costs varied considerably. The...

Study smarter with the SolutionInn App


