Question: Use ionic radii data (Resource section 1) to suggest possible dopants to increase anion conductivity in (a) PbF 2 (b) Bi 2 O 3 (six-coordinate
Use ionic radii data (Resource section 1) to suggest possible dopants to increase anion conductivity in
(a) PbF2
(b) Bi2O3 (six-coordinate Bi3+).
Resource section 1.
Ionic radii are given (in picometres, pm) for the most common oxidation states and coordination geometries. The coordination number is given in parentheses, (4) refers to tetrahedral and (4SP) refers to square planar. All d-block species are low-spin unless labelled with †, in which case values for high-spin are quoted. Most data are taken from R.D. Shannon, Acta Crystallogr., 1976, A32, 751, where values for other coordination geometries can be found. Where Shannon values are not available, Pauling ionic radii are quoted and are indicated by *.
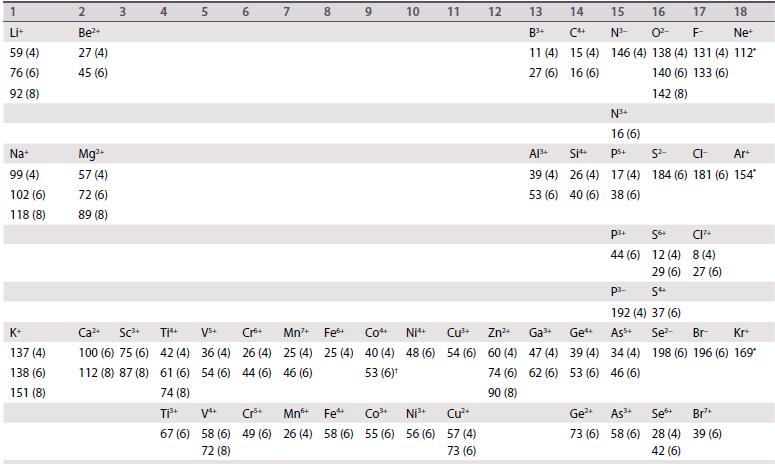
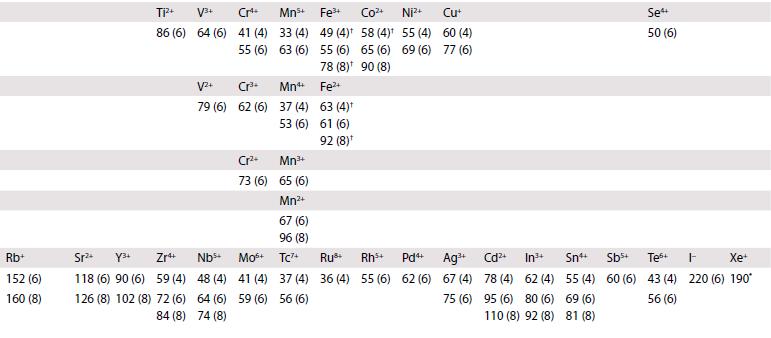
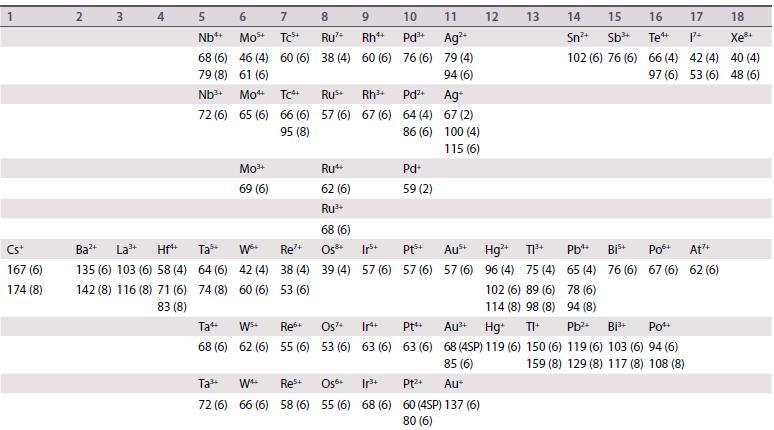
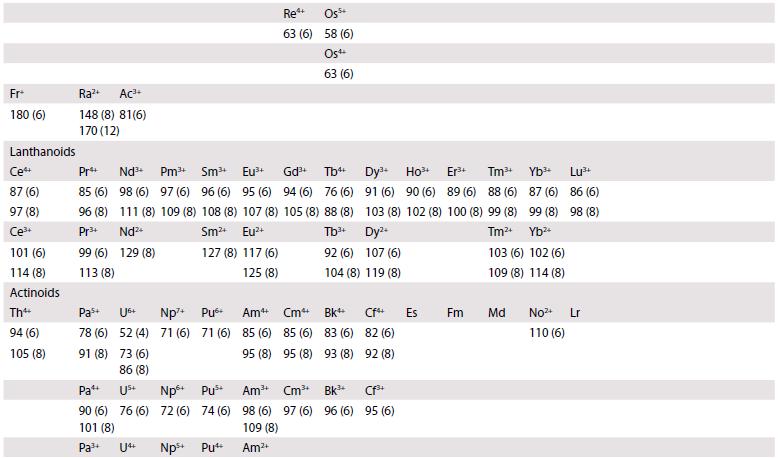

1 Li+ 59 (4) 76 (6) 92 (8) Na+ 99 (4) 102 (6) 118 (8) K+ 137 (4) 138 (6) 151 (8) 2 Be2+ 27 (4) 45 (6) Mg2+ 57 (4) 72 (6) 89 (8) 3 4 Ca+ Sc+ Ti 100 (6) 75 (6) 112 (8) 87 (8) 42 (4) 61 (6) 74 (8) 5 Vs+ 36 (4) 54 (6) 67 (6) 58 (6) 72 (8) 60 Cr 26 (4) 44 (6) Cr+ 49 (6) 7 8 Mn+ Fe+ 25 (4) 25 (4) 46 (6) 9 Co4 40 (4) 53 (6) 10 11 Ni Cu+ 48 (6) 54 (6) Mn+ Fe Ni+ 26 (4) 58 (6) 55 (6) 56 (6) Cu+ 57 (4) 73 (6) 12 Zn+ 60 (4) 74 (6) 90 (8) 13 14 B+ (4 11 (4) 15 (4) 27 (6) 16 (6) A1+ Si4+ 39 (4) 26 (4) 53 (6) 40 (6) Ga+ 47 (4) 62 (6) Ge 39 (4) 53 (6) 15 16 17 18 N- 0- F- Ne 146 (4) 138 (4) 131 (4) 112* 140 (6) 133 (6) 142 (8) N+ 16 (6) ps+ 17 (4) 38 (6) P+ 44 (6) 5- CI- Ar+ 184 (6) 181 (6) 154* Ge4 As+ 73 (6) 58 (6) 56+ 12 (4) 29 (6) S4+ P- 192 (4) 37 (6) Ass+ 34 (4) 46 (6) CI7+ 8 (4) 27 (6) Se- Br- Kr+ 198 (6) 196 (6) 169* Se 28 (4) 42 (6) Br7+ 39 (6)
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
To suggest possible dopants to increase anion conductivity in the given compounds we should consider elements that can substitute for the cations in t... View full answer

Get step-by-step solutions from verified subject matter experts


