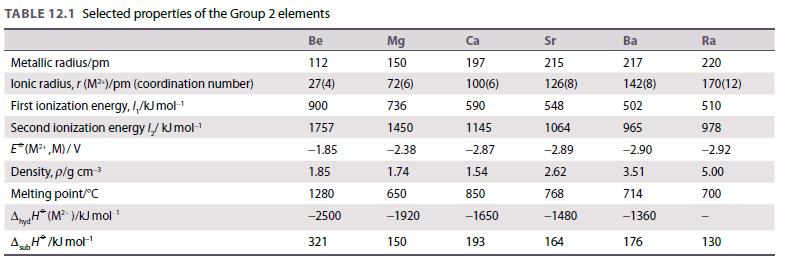
Use your knowledge of chemical trends in Group 2 chemistry and data from Table 12.1 to predict
Question:
Use your knowledge of chemical trends in Group 2 chemistry and data from Table 12.1 to predict the chemistry of radium. Compare your predictions with experimental observations. See, for example, H.W. Kirby and M.L. Salutsky, The radiochemistry of radium. Nuclear Science Series, National Academy of Sciences, National Research Council. National Bureau of Standards, US Department of Commerce (1964). http://library.lanl.gov/cgi-bin/getfile?rc000041.pdf (accessed January 2018).
Table 12.1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





