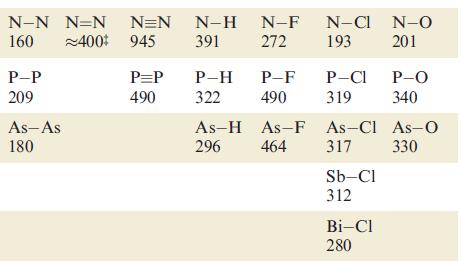
Using bond enthalpy terms from Tables 14.2 and 15.3, estimate values of r H for the
Question:
Using bond enthalpy terms from Tables 14.2 and 15.3, estimate values of ΔrHº for the following reactions
(a) 2N2 → N4 (tetrahedral structure);
(b) 2P2 → P4 (tetrahedral structure);
(c) 2C2H2 → C4H4 (tetrahedrane, with a tetrahedral C4 core).
Table 14.2.
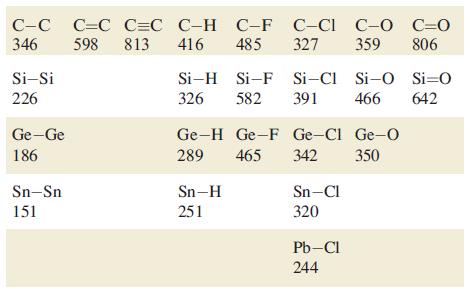
Table 15.3.
Transcribed Image Text:
C-C_C=CC=C_C-H C-F C-CI C-0_C=0 598 813 416 485 327 359 806 346 Si-Si 226 Ge-Ge 186 Sn-Sn 151 Si-H Si-F Si-Cl Si-O Si=0 582 391 466 326 642 Ge-H Ge-F 289 465 342 350 Ge-Cl Ge-0 Sn-H 251 Sn-Cl 320 Pb-Cl 244
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To estimate the values of rH standard enthalpy change for the given reactions using bond enthalpy te...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using data from Appendix 4, calculate ÎHo, ÎSo, and DGo for the following reactions that produce acetic acid: Which reaction would you choose as a commercial method for producing acetic...
-
For the following reactions at constant pressure, predict if H . E, H , E, or H = E. a. 2HF(g) H2(g) + F2(g) b. N2(g) + 3H2(g) 2NH3(g) c. 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
-
Using the data in Appendix 3, calculate the standard entropy changes for the following reactions at 25C: (a) S(s) + O2(g) SO2(g) (b) MgCO3(s) MgO(s) + CO2(g)
-
Consider each of the following situations. Indicate whether (and why or why not) you think that the governement should account for the transactions and resources in an agency fund, a governmental...
-
A number of business transactions carried out by Smalling Manufacturing Company are as follows: a. Borrowed money from a bank. b. Sold land for cash at a price equal to its cost. c. Paid a liability....
-
In your opinion, what are 5 key activities that promote software quality? How will these activities promote quality and make the project a success? What are challenges you might encounter...
-
On January 1, 2009, Morey, Inc., exchanged $178,000 for 25 percent ofAmsterdam Corporation. Morey appropriately applied the equity method to this investment. At January 1, the book values of...
-
The Atlas Company sells product T. During a move to a new location, the inventory records for product T were misplaced. The bookkeeper has been able to gather some information from the sales records...
-
I need help of 5 & 6
-
Lead-acid batteries accounted for 69% of all lead consumed in the US in 2015. (a) Complete the cell reaction given below (not balanced on the left-hand side) and show that the oxidation state changes...
-
The glass industry manufactures millions of tonnes of glass per year. (a) Only certain element oxides form glasses. Explain why this is, giving examples of what are termed in the glass industry as...
-
Data for Stojanovic Distributing Company are presented in P5.5B. A physical inventory count shows the company has $5,570 of inventory on hand at September 30, 2024. Instructions a. Record the...
-
In this problem, we consider mild modifications of the standard MDP setting. (a) (10 points) Sometimes MDPs are formulated with a reward function R(s) that depends only on the current state. Write...
-
All-Walnut, Inc. produces two models of bookcases. The bookcases sell for the amount listed in the table below. Each bookcase requires a certain number of labor hours, machine time, and materials...
-
Problem 1 Find the number of degrees of freedom of the mechanisms (a)-(d) (a) (b)
-
3) (10 pts) The following grammar is given E EAE (E) -E | id V={E,A), T={-,(,),*,/,+,id} and starting symbol is E. a) Give the left-most derivation of w= id+id*id. Is w accepted? b) Is this a...
-
4. X, the proprietor of a departmental store, decided to calculate separate profits for his two departments L and M for the month ending 31st January. Stock on 31st January could not be valued for...
-
Explain the difference between savings and investment as defined by macroeconomists. Which of the following situation represent investment? Saving? Explain. Savings is that part of income which is...
-
Highland Theatre is owned by Finnean Ferguson. At June 30, 2014, the ledger showed the following: Cash, $6,000; Land, $100,000; Buildings, $80,000; Equipment, $25,000; Accounts Payable, $5,000;...
-
Draw the B 12 unit that is a common motif of boron structures; take a viewpoint along a C 2 axis.
-
Give balanced chemical equations for the synthesis of 1,2-B 10 C 2 H 10 (Si(CH 3 ) 3 ) 2 starting with decaborane(14) and other reagents of your choice.
-
Give the IUPAC names of (a) B 10 H 14 , (b) [B 12 H 12 ] 2 , (c) Arachno-[B 12 H 14 ] 2 .
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


