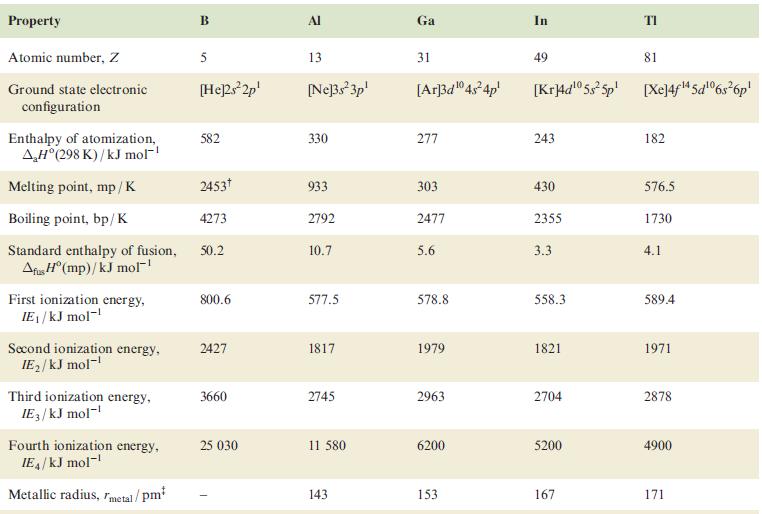
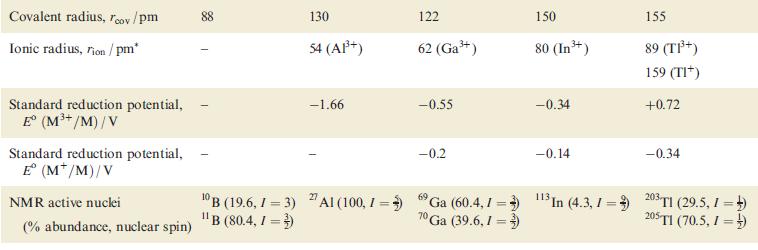
Using the data in Table 13.1, draw a potential diagram for Tl and determine the value of
Question:
Using the data in Table 13.1, draw a potential diagram for Tl and determine the value of Eº(Tl3+/Tl+).
Table 13.1
Transcribed Image Text:
Property Atomic number, Z Ground state electronic configuration Enthalpy of atomization, AH°(298 K)/kJ mol-¹ Melting point, mp/K Boiling point, bp/K Standard enthalpy of fusion, Afus H° (mp)/kJ mol™¹ First ionization energy, IE₁/kJ mol-¹ Second ionization energy, IE₂/kJ mol-¹ Third ionization energy, IE3/kJ mol-¹ Fourth ionization energy, IE4/kJ mol-¹ Metallic radius, metal/pm² B 5 [He]2s²2p¹ 582 2453 4273 50.2 800.6 2427 3660 25 030 Al 13 [Ne]3s²3p¹ 330 933 2792 10.7 577.5 1817 2745 11 580 143 Ga 31 [Ar]3d¹04s²4p¹ 277 303 2477 5.6 578.8 1979 2963 6200 153 In 49 81 [Kr]4d¹05s²5p [Xe]4f¹4 5d¹06s²6p¹ 243 430 2355 3.3 558.3 1821 2704 5200 167 182 576.5 1730 4.1 589.4 1971 2878 4900 171
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
The given data from Table 131 includes the standard reduction potentials for various elements including thallium Specifically we are provided with the following standard reduction potentials Tl3 2e Tl ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The data in Table P-13 are closing stock quotations for the DEF Corporation for 150 days. Determine the appropriate ARIMA model, and forecast the stock price five days ahead from forecast origin t =...
-
Using the data in Table 12.29, perform a multiple comparisons procedure to identify which specific underlying means are different? Nutrition Researchers compared protein intake among three groups of...
-
Using the data in Table 12.5, do the following: (a) Determine the flexural strength for nonporous MgO assuming a value of 3.75 for n in Equation 12.10. (b) Compute the volume fraction porosity at...
-
Joseph Thompson is president and sole shareholder of Jay Corporation. In December 2019, Joe asks your advice regarding a charitable contribution he plans to have the corporation make to the...
-
Explain the following terms: recovery of investment versus return on investment.
-
State the definition of continuity of a vector-valued function. Give an example of a vector-valued function that is defined but not continuous at t = 2.
-
Senthil Construction Company undertook a contract for constructing a building from 1st January 1998. The contract price was Rs 1,00,000. He incurred the following expenses. Rs Materials issued 6,000...
-
Slick Pads is a company that manufactures laptop notebook computers. The company is considering adding its own line of computer printers as well. It has considered the implications from the marketing...
-
Ayla, age 17, is claimed by her parents as a dependent. During 2020, she had interest income from a bank savings account of$ 2,000 and income from a part-time job of$ 4,200. Ayla's taxable income is:...
-
Plot a graph to show the variation in values of IE 1 , IE 2 and IE 3 for the group 13 elements (Table 13.1), and plot a similar graph to show the variation in values of IE 1 and IE 2 for the group 2...
-
World production of lime in 2014 was 360 Mt. The term lime may refer to CaO (quicklime) and/or slaked lime (Ca(OH) 2 ), but is also used to include CaO, Ca(OH) 2 , CaOMgO, Ca(OH) 2 MgO and Ca(OH) 2...
-
Why is English the leading language of international corporate financial reporting?
-
2. (40 marks) Solve for y(t) such that y" - 6y' + 15y = 2 sin(3t),
-
6. Determine output class A{ ); } public static void main(String args[]) { int x; x = 10; if (x == 10) { int y = 20; System.out.print ("x and y: y = x*2; + y); } y = 100; } System.out.print ("x and...
-
Anita and Bonita have been roommates for the past two years while they've been in graduate school. Now that they're graduating, they are each planning to move to different cities. Their one joint...
-
To what extent are business ethics assumed, or taken for granted, by people in businesses?
-
Empowered by what he has learned in this class about gender, Brady makes a friendly wager with his girfriend, Marlisa: "I bet I can guess how the men and women at the next table will behave during...
-
If you borrow at 6% per year for 5 years and use the proceeds to buy a 5 year bond that has a yield to maturity of 8% your leveraged position is doomed because of credit risk. (Assume that all bonds...
-
A line l passes through the points with coordinates (0, 5) and (6, 7). a. Find the gradient of the line. b. Find an equation of the line in the form ax + by + c = 0.
-
Draw the bcc unit cell of tungsten metal and add a second neighbouring unit cell. What, approximately, is the CN of a site in the face of the original unit cell? What would be the stoichiometry of a...
-
In the structure of MoS 2 , the S atoms are arranged in closepacked layers that repeat themselves in the sequence AAA . . .. The Mo atoms occupy holes with coordination number 6. Show that each Mo...
-
Metallic copper adopts an fcc structure with density 8960 kg m 3 . Draw the unit cell of copper and mark the shortest copper atom to copper atom distance. How many copper atoms are there in the unit...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


