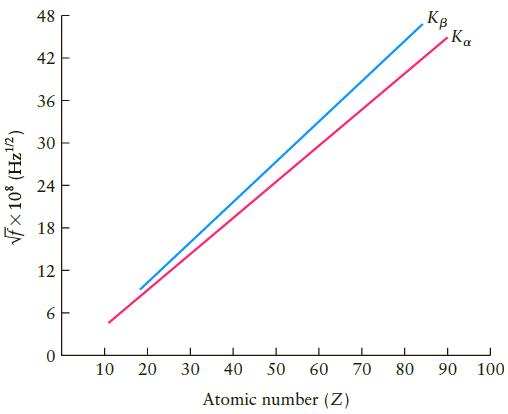
Referring to Figure10.36, we see that the atomic number Z is proportional to f 1/2 or that
Question:
Referring to Figure10.36, we see that the atomic number Z is proportional to f1/2 or that Z2 is proportional to f. Because the frequency of the characteristic x-ray lines is itself proportional to the energy of the associated x-ray photon, we are led to conclude that DE and hence the energies of the atomic levels also scale as Z2. Based on this analysis (and ignoring any differences from the masses of their nuclei), how much greater is the energy associated with the ground state of helium (Z = 2) than that of hydrogen (Z = 1)?
Figure 10.36
 Make a similar comparison between the ground state energies for sodium (Z = 11) and hydrogen.
Make a similar comparison between the ground state energies for sodium (Z = 11) and hydrogen.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





