Use MuPAD to compute the following limits. a. lim - 4 b. lim x--2 + 4 x4
Question:
Use MuPAD to compute the following limits.
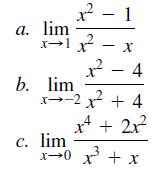
Transcribed Image Text:
a. lim - 4 b. lim x--2 + 4 x4 + 2x? c. lim x-0 x + x
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use MuPAD to compute the following limits. a. lim x* x0+ b. lim (cos x)/tan x x0+ 1 1- c. lim x-0+ sin x d. lim x-0- r 2 - 25 e. lim x-5-x - 10x +25 x - 1 f. lim x1+ sin[(x - 1)']
-
Use MuPAD to compute the following limits. x + 1 lim x- 00 . 3x b. lim 2x 2x + 3 X -00
-
Use integration by partial fractions to compute the following indefinite integrals. 2
-
Two stocks, A and B, have beta coefficients of 0.8 and 1.4, respectively. If the expected return on the market is 10 percent and the risk-free rate is 5 percent, what is the risk premium associated...
-
Based on the following reaction profile, how many intermediates are formed in the reaction A -- C? How many transition states are there? Which step is the fastest? Is the reaction A -- C exothermic...
-
Describe the phases of the business research process.
-
Consistency? Table 10.2 shows how Indiana state lottery sales are divided among different types of games. What is the sum of the amounts spent on the nine types of games? Why is this sum not exactly...
-
Operating budgets: labor hiring plan Strathfield Motel is planning its operations for the upcoming tourist season. The motel has 60 units. The following table presents the average number of daily...
-
E23.9 (L02,3) (SCF-Direct Method) Costa SA uses the direct method to prepare its statement on cash flows. Costa's trial balances at December 31, 2019 and 2018, are as follows. 23-46 CHAPTER 23...
-
Suppose you are asked to make a loan for 10% from one year for now; you decided to compare interest rates with Government bonds and make at least 2% premium over that. One-year bond has 5% yield and...
-
Integrals that cannot be evaluated in closed form sometimes can be evaluated approximately by using a series representation for the integrand. For example, the following integral is used for some...
-
Find the expression for the sum of the geometric series for r 1. n-1 k=0
-
Suppose 50.0 mL of 0.250 M CoCl 2 solution is added to 25.0 mL of 0.350 M NiCl 2 solution. Calculate the concentration, in moles per liter, of each of the ions present after mixing. Assume that the...
-
Swenson Company produced 300 units in year one and sold 260 units in that year. In year two, it produced 260 units and sold 300 units. Total fixed overhead was the same in years one and two. Under...
-
c) Determine the maximum rotational speed such that the fluid will not spill over the container. (and: = 2gh/R) [2 marks] d) The container in Figure 4 now contains coffee (p~1000) which is 7cm deep...
-
FICO credit scores: x = 564,= 743,= 72 (Round your answer to 3 decimal places.) what does z equal
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28 W/m. 'C. The thermal conductivity of the solid material is...
-
25 of 27 > This test: 96 point(s) possible This question: 3 point(s) possible Submit test Identical twins come from a single egg that split into two embryos, and fraternal twins are from separate...
-
Find the transfer function, G(s) = X 1 (s)/F(s), for the translational mechanical system shown in Figure P2.9. -x(1) 4 N-s/m 5 N/m 5 kg FIGURE P2.9
-
The executor of Gina Purcells estate has recorded the following information: Assets discovered at death (at fair value): Cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
A solution with an ionic strength of 0.10 M containing 0.010 0 M phenylhydrazine has a pH of 8.13. Using activity coefficients correctly, find pKa for the phenylhydrazinium ion found in...
-
Use the Goal Seek spreadsheet at the end of the chapter to find the pH of 1.00 L of solution containing 0.030 mol HA (pK a = 2.50) and 0.015 mol NaA. What would the pH be with the approximations [HA]...
-
Why doesnt water dissociate to produce 10 -7 M H + and 10 -7 M OH - when some HBr is added?
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App



