An engineer claims that the volume expansity of an ideal solution is given by Is this claim
Question:
An engineer claims that the volume expansity of an ideal solution is given by
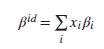
Is this claim valid? If so, show why. If not, find a correct expression for βid.
Transcribed Image Text:
pid = Exißi %3D i
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
The claim is not valid The correct expression for the ideal solutions volume expansivity id is g...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Someone claims that the volume flow rate in a circular pipe with laminar flow can be determined by measuring the velocity at the centerline in the fully developed region, multiplying it by the...
-
An automobile safety engineer claims that 1 in 10 automobile accidents is due to driver fatigue. Using the formula for the binomial distribution and rounding to four decimals, what is the probability...
-
An experiment is conducted so that, an engineer can gain insight into the influence of sealing temperature A, cooling bar temperature B, percent polyethylene additive C, and pressure D on the seal...
-
Ethelbert.com is a young software company owned by two entrepreneurs. It currently needs to raise $400,000 to support its expansion plans. A venture capitalist is prepared to provide the cash in...
-
1. List the benefits of globalization. 2. List the costs of globalization.
-
Arcola, Inc., acquires all 40,000 shares of Tuscola Company for $725,000. A year later, when Arcolas equity adjusted balance in its investment in Tuscola equals $800,000, Tuscola issues an additional...
-
Identify the types of selection errors and biases managers must overcome when interviewing job applicants. (pp. 137-140)
-
On July 1, 2017, Amos Corporation granted nontransferable, nonqualified stock options to certain key employees as additional compensation. The options permit the purchase of 20,000 shares of Amos's...
-
Question 4 10 pts Company W's current income statement reflects the following information: Sales volume = 25,000, sale price = $15.00, per unit variable expenses = $5.00, and total fixed expenses =...
-
The following selected accounts and their current balances appear in the ledger of Clairemont Co. for the fiscal year ended May 31, 2019: Selected accounts and related amounts for Clairemont Co. for...
-
Laboratory A reports the following results for equimolar values of G E for liquid mixtures of benzene(1) with 1-hexanol(2): G E = 805 Jmol 1 at T = 298 K G E = 785 Jmol 1 at T = 323 K Laboratory B...
-
The following empirical two-parameter expression has been proposed for correlation of excess properties of symmetrical liquid mixtures: Here, quantities A and B are parameters that depend at most on...
-
Suppose that the range of the continuous variables X and Y is 0 < x < a and 0 < y < b. Also suppose that the joint probability density function f XY (x, y) = g(x)h(y), where g(x) is a function only...
-
Suppose that your credit card activity for December looked like this: Date Activity December 5 $384 purchase December 11 $347 purchase December 16 $174 purchase December 21 $480 purchase December 25...
-
A research group surveyed 300 students. The students were asked how often they go to the movies and whether they prefer comedies or dramas. Their responses are summarized in the following table....
-
C. Prove the following (you can use any formal induction/other theoretical method, "A" means power here): i. ii. iii. What is the time complexity recurrence relation for Fibonacci numbers? Explain it...
-
You and your partner run a small business together, with separate work roles. You are responsible for the business budget and have researched an improved budget process which you felt needs to be...
-
The actual selling expenses incurred in March 2022 by Carla Vista Company are as follows: Variable Expenses Fixed Expenses Sales commissions Advertising $14,576 Sales salaries $34,700 12.174...
-
On March 1, 2019, Elkhart enters into a new contract to build a specialized warehouse for $7 million. The promise to transfer the warehouse is determined to be a performance obligation. The contract...
-
Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cd2+ (b) p3- (c) Zr4+ (d) Ru3+ (e) As3- (f) Ag+
-
SO 2 vapor enters a heat exchanger at 100C and at a flowrate of 45 mole/h. If heat is transferred to the SO 2 at a rate of 1,300 kJ/h, what is the rate of entropy transport in the gas at the outlet...
-
Operating a wind tunnel for aircraft experiments begins with adiabatically and reversibly compressing atmospheric air (300 K) into long cylinders comprising a total volume of 20 m 3 at 200 bars. The...
-
An ideal gas enters a valve at 500 K and 3 MPa at a steady-state rate of 3 mol/min. It is throttled to 0.5 MPa. What is the rate of entropy generation? Is the process irreversible?
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


