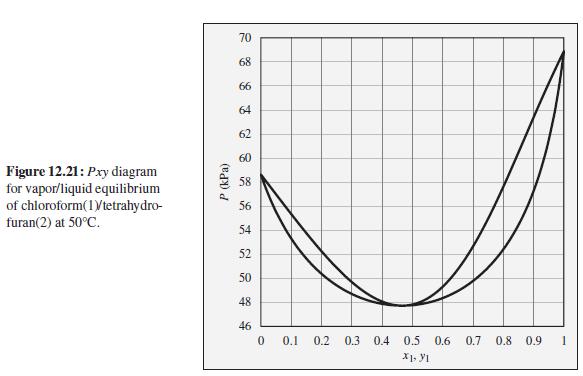
Consider a chloroform (1)/tetrahydrofuran(2) mixture with x 1 = 0.80, initially at 50C and 70 kPa. Describe
Question:
Consider a chloroform (1)/tetrahydrofuran(2) mixture with x1 = 0.80, initially at 50°C and 70 kPa. Describe the evolution of phases and phase compositions as the pressure is gradually reduced to 50 kPa.
To the Pxy diagram for chloroform(1)/tetrahydrofuran(2) at 50°C shown in Fig. 12.21.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





