Estimate the standard Gibbs free energy and the equilibrium constant at 1 bar and (800 mathrm{~K}) for
Question:
Estimate the standard Gibbs free energy and the equilibrium constant at 1 bar and \(800 \mathrm{~K}\) for the reaction
\[ \mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g}) \]
Given that the standard heat of reaction, \(\Delta H_{298}^{0}\), and standard Gibbs free energy of the reaction, \(\Delta G_{298}^{0}\), for the formation of products are \(-92.11 \mathrm{~kJ} / \mathrm{mol}\) and \(-33.494 \mathrm{~kJ} / \mathrm{mol}\) respectively. The specific heat data in \(\mathrm{kJ}\) as function of temperature are as follows: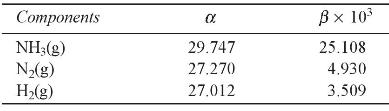
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





