Figure 3.3 suggests that the isochores (paths of constant volume) are approximately straight lines on a P-T
Question:
Figure 3.3 suggests that the isochores (paths of constant volume) are approximately straight lines on a P-T diagram. Show that the following models imply linear isochores.
(a) Constant-β, κ equation for liquids
(b) Ideal-gas equation
(c) Van der Waals equation
Figure 3.3
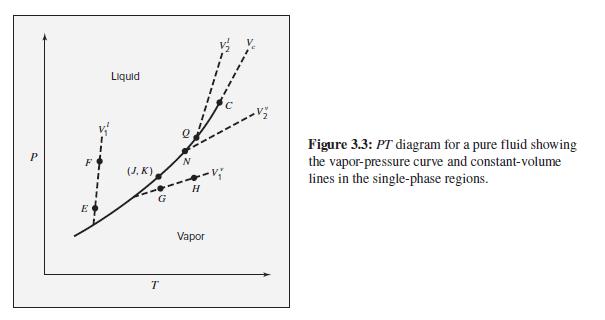
Transcribed Image Text:
Liquid Figure 3.3: PT diagram for a pure fluid showing the vapor-pressure curve and constant-volume lines in the single-phase regions. (J, K) G E Vapor T.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
Isochores constant volume lines are approximately straight lines on a PT diagram for some fluids We ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
A certain gas obeys the van der Waals equation with a =0.76 m6 Pa mol-2, its volume is found to be 4.00 X 10-4 m3 mol-1 at 288 K and 4.0 MPa. From this information calculate the van der Waals...
-
Show that the van der Waals equation can be written as a cubic equation in the compressibility factor involving the reduced pressure and reduced temperature as 27 P 2 512T 3
-
Read each case carefully and, as determined in the Internal Revenue Code of Puerto Rico, identify the deductions, calculate the amount of deductions and what the determined contribution would be. and...
-
Why do you think companies invest resources in training their managers and employees in business ethics? Some people think that ethics should be learned within the family and by the time a person is...
-
Why can the book value and market value of a firm differ?
-
Use Analytic Solver Platform to simulate the Outsourcing Decision Model under the assumptions that the production volume will be triangular with a minimum of 800, maximum of 1,700, and most likely...
-
What criteria must be met before donated services can be recorded as contribution revenue and an expense? Give an example of a service that might qualify as a donated service for accounting purposes.
-
Bramble Products manufactures and sells a variety of camping products. Recently the company opened a new factory to manufacture a deluxe portable cooking unit. Cost and sales data for the first month...
-
Zippy Cola is studying the effect of its last advertising campaign. People chosen at random were called and asked how many cans of zippy cola they hand bought and advertisements they had either read...
-
For a gas described by the Redlich/Kwong equation and for a temperature greater than T c , develop expressions for the two limiting slopes, Note that in the limit as P 0, V , and that in the limit...
-
Show that the density-series second virial coefficients can be derived from isothermal volumetric data via the expression: B = lim(Z 1)/p p-0 p(molar density)= 1/V -
-
The manager of a paint supply store wants to estimate the actual amount of paint contained in 1-gallon cans purchased from a nationally known manufacturer. The manufacturer's specifications state...
-
Question: Divide.21r7-35r37r3r8-5r43r7-5r33r6-5r221r6-35r2 Divide. 2 1 r7 - 3 5 r3 7r 3r 8 - 5 r4 3 r7 - 5 r3 3 r6 - 5 r2 2 1 r6 - 3 5 r2 Divide. 21r7 - 35r 7r 38-54 3r7-53 3r6-52 216-3512
-
System of Equations: Value of a Value of b 9 a + 3 b = 3 0 8 a + 4 b = 2 8
-
An important practice is to check the validity of any data set that you analyze. One goal is to detect typos in the data, and another would be to detect faulty measurements. Recall that outliers are...
-
ve The re e Problem 3 Complete the following perpetual inventory form. Perpetual Inventory Total Product Name: Purchase Unit Size: Carried Forward: Date In Out 1/7 3 Balance 1/9 1/10 1/12 1/15 2 1 5...
-
the above date, Saloni was admitted in the partnership firm. Raman surrendered th 5 2 of his share and Rohit surrendered th 5 1 of his share in favour of Saloni. It was agreed that : (i) Plant and...
-
Timmer Company signs a lease agreement dated January 1, 2019, that provides for it to lease equipment from Landau Company beginning January 1, 2019. The lease terms, provisions, and related events...
-
On April 29, 2015, Auk Corporation acquires 100% of the outstanding stock of Amazon Corporation (E & P of $750,000) for $1.2 million. Amazon has assets with a fair market value of $1.4 million (basis...
-
For a binary gas mixture described by Kqs, (3,381 and (l l.62l. prove that: See also Eq, (11.87), and note that δ12 = 2B12 - B11 - B22. ds1g d2812 PyIV2 dT
-
What is the ideal work for the separation of an equimolar mixture of methane and ethane at 175C and 3 bar in a steady-flow process into product streams of the pure gases at 35C and 1 bar if Ta = 300...
-
What is the work required for the separation of air (21-mol-% oxygen and 79-mol-% nitrogen) at 25C and 1 bar in a steady-flow process into product streams of pure oxygen and nitrogen, also at 25C and...
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...
-
Which of the following is a limitation of both return on investment and residual income? A. Favors large units. B. There is disincentive for high return on investment units to invest. C. Can lead to...

Study smarter with the SolutionInn App


