For methanol synthesis reaction [ mathrm{CO}(mathrm{g})+2 mathrm{H}_{2}(mathrm{~g}) ightleftharpoons mathrm{CH}_{3} mathrm{OH}(mathrm{g}) ] Using a feed mixture of
Question:
For methanol synthesis reaction
\[ \mathrm{CO}(\mathrm{g})+2 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons \mathrm{CH}_{3} \mathrm{OH}(\mathrm{g}) \]
Using a feed mixture of carbon monoxide and hydrogen in the stoichiometric proportion, state what equilibrium mole fraction of methanol at 50 bar pressure and \(450 \mathrm{~K}\) can be found from the following data: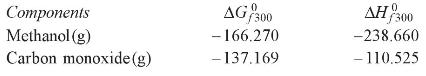
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





