For the acetone(1)/methanol(2)/water(3) system, based on Eq. (13.19) and the Wilson equation, make the following calculations: (a)
Question:
For the acetone(1)/methanol(2)/water(3) system, based on Eq. (13.19) and the Wilson equation, make the following calculations:
(a) BUBL T : P = 101.33 kPa, x1 = 0.3, x2 = 0.4.
(b) DEW T : P = 101.33 kPa, y1 = 0.3, y2 = 0.4.
(c) P, T − flash : P = 101.33 kPa, T = ½ (Tbubble + Tdew), z1 = 0.3, z2 = 0.2.
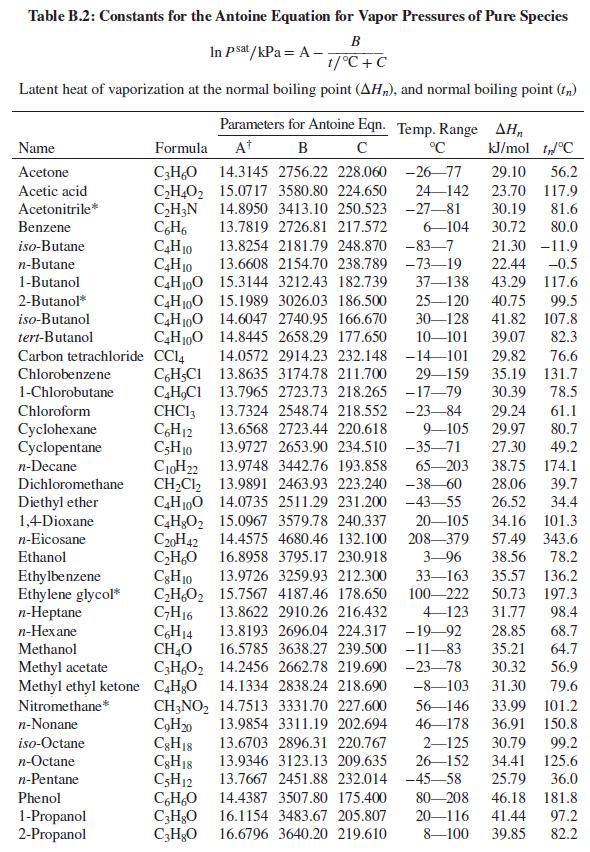
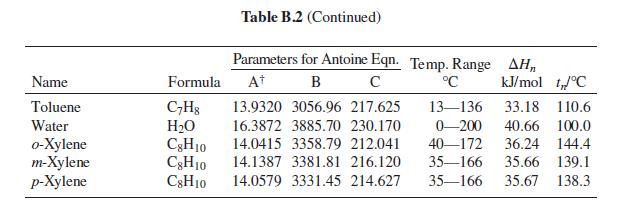
Problems 13.43 through 13.54 require parameter values for the Wilson or NRTL equation for liquid-phase activity coefficients. Table 13.10 gives parameter values for both equations. Antoine equations for vapor pressure are given in Table B.2, Appendix B.
Table 13.10
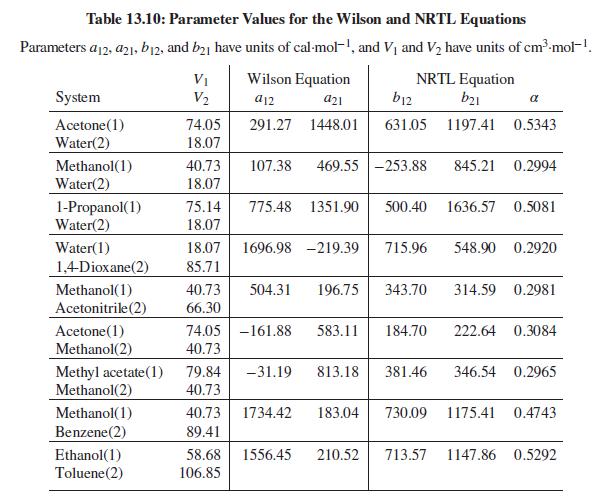
Table B.2, Appendix B.
Eq. (13.19)
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





