For the benzene-toluene system, (z_{1}=0.81) at (60^{circ} mathrm{C}) and (70 mathrm{kPa}). The vapour pressure of the components
Question:
For the benzene-toluene system, \(z_{1}=0.81\) at \(60^{\circ} \mathrm{C}\) and \(70 \mathrm{kPa}\). The vapour pressure of the components are given by the Antoine equation \(\ln P=A-\frac{B}{T+C}\), where \(T\) is in \({ }^{\circ} \mathrm{C}\) and \(P\) is in \(\mathrm{kPa}\). Antoine constants for the components are as follows: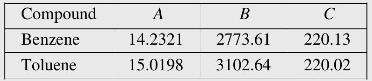
Determine the moles and composition of the vapour and liquid streams \(V, L\) and \(x, y\) respectively.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





