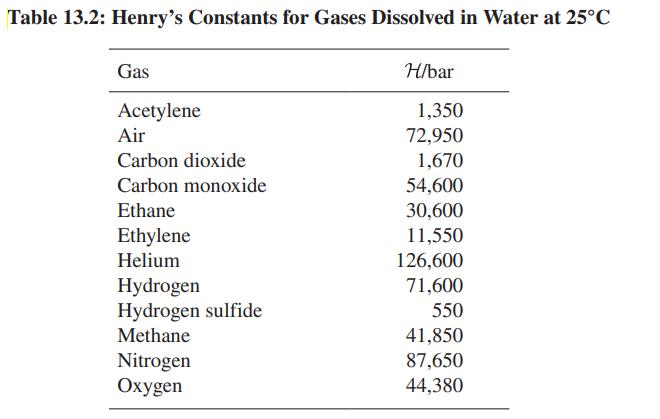
Helium-laced gases are used as breathing media for deep-sea divers. Why? Table 13.2 may provide useful data.
Question:
Helium-laced gases are used as breathing media for deep-sea divers. Why? Table 13.2 may provide useful data.
Transcribed Image Text:
Table 13.2: Henry's Constants for Gases Dissolved in Water at 25°C Gas Acetylene Air Carbon dioxide Carbon monoxide Ethane Ethylene Helium Hydrogen Hydrogen sulfide Methane Nitrogen Oxygen H/bar 1,350 72,950 1,670 54,600 30,600 11,550 126,600 71,600 550 41,850 87,650 44,380
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Heliumlaced gases are used as breathing media for deepsea divers for several important reasons primarily related to its low solubility in blood and ti...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781260597684
9th International Edition
Authors: J.M. Smith, Mark Swihart Hendrick C. Van Ness, Michael Abbott
Question Posted:
Students also viewed these Engineering questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
On June 23, 2018, in Thailand, a group of 12 boys aged between 11 and 17 from the local football team, named the Wild Boars, and their 23-year-old assistant coach entered the Tham Luang cave. Tham...
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
Attijari Wafa Bank distributed on May 2022 a dividend per share of 5 MAD / share. The company is planning to have next 3 years a growth rate of 5% then after 7%. If the required rate of return is 12%...
-
Assume the correlation coefficient between Baker Fund and the S&P 500 Stock Index is .70. What percentage of Baker Funds total risk is specific (i.e., nonsystematic)?
-
When a by-product has an established market, what items should be deducted from the market price to arrive at the by-product valuation? LO.1
-
What are organization expenses? Provide examples. AppendixLO1
-
Xellnet provides e- commerce software for the pharmaceuticals industry. Xellnet is orga-nized into several divisions. A companywide planning committee sets general strategy and goals for the company...
-
please i need help Vilas Company is considering a capital investment of $216.000 in additional productive facilities. The new machinery is expected to luvallife of years with no salvage value....
-
In the Reddy Mikks model of Example 2.2-1; (a) Determine the range for the ratio of the unit revenue of exterior paint to the unit revenue of interior paint. (b) If the revenue per ton of exterior...
-
A system formed initially of 2 mol CO 2 , 5 mol H 2 , and 1 mol CO undergoes the reactions: Develop expressions for the mole fractions of the reacting species as functions of the reaction coordinates...
-
Which is the more effective way to increase the coefficient of performance of a Carnot refrigerator: to increase T C with T H constant, or to decrease T H with T C constant? For a real refrigerator,...
-
The following information pertains to Godwin Motors, which uses the allowance method for receivables: Required a. Assuming that Godwin Motors recorded bad debt expense of $25,600 in 2012, what amount...
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
You have been asked to review the December 31, 2018, balance sheet for champion cleaning. After completing your review, you list the following three items for discussion with your superior: 1. An...
-
Linda Lopez opened a beauty studio, Lindas Salon, on January 2, 2011. The salon also sells beauty supplies. In January 2012, Lopez realized she had never filed any tax reports for her business and...
-
A preliminary evaluation of a new process concept has produced a waste stream of the composition given below. It is desired to reduce the waste stream to 10% of its original mass while recovering...
-
According to Gmehling et al. (1994),23 the system acetone + water shows azeotropes at: (1) 2793 mmHg, 95.1 mol% acetone, and 100C; and (2) 5155 mmHg, 88.4 mol% acetone and 124C. What azeotropic...
-
Activity coefficients are an implicit part of the equation of state but they can be determined explicitly by comparing the definitions of the K-ratios. Using the k ij value fit at x e = 0.415,...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


