Humidity, relating to the quantity of moisture in atmospheric air, is accurately given by equations derived from
Question:
Humidity, relating to the quantity of moisture in atmospheric air, is accurately given by equations derived from the ideal-gas law and Raoult’s law for H2O.
(a) The absolute humidity h is defined as the mass of water vapor in a unit mass of dry air. Show that it is given by:
![]()
where ℳ represents a molar mass and pH2O is the partial pressure of the water vapor, i.e., pH2O = yH2OP .
(b) The saturation humidity hsat is defined as the value of h when air is in equilibrium with a large body of pure water. Show that it is given by:
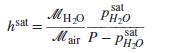
where psatH2O is the vapor pressure of water at the ambient temperature.
(c) The percentage humidity is defined as the ratio of h to its saturation value, expressed as a percentage. On the other hand, the relative humidity is defined as the ratio of the partial pressure of water vapor in air to its vapor pressure, expressed as a percentage. What is the relation between these two quantities?
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





