Show how the general rate form of the entropy balance, Eq. (5.16), reduces to Eq. (5.2) for
Question:
Show how the general rate form of the entropy balance, Eq. (5.16), reduces to Eq. (5.2) for the case of a closed system.
Eq. (5.16)
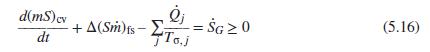
Eq. (5.2)
![]()
Transcribed Image Text:
d(mS)cv + A(Sm)fs- E = ŠG 2 0 To.j (5.16) dt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
To show how the general rate form of the entropy balance Eq 516 reduces to Eq 52 for the case of a c...View the full answer

Answered By

Chandrasekhar Karri
I have tutored students in accounting at the high school and college levels. I have developed strong teaching methods, which allow me to effectively explain complex accounting concepts to students. Additionally, I am committed to helping students reach their academic goals and providing them with the necessary tools to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Customized software products Select one: O a. Software must be acceptable to any type of users. O b. The specification of what the software should do is owned by the customer for the software and...
-
A closed system receives heat at a rate of 2 kW from a source at 1000 K, electrical power at a rate of 2 kW and shaft power at a rate of 2 kW. Determine the net rate of (a) Energy transfer and (b)...
-
How does a flexible exchange rate system in general adjust balanceofpayments disequilibria? How does a fixed exchange rate system in general adjust balanceofpayments disequilibria? Why is the choice...
-
NIU Company's budgeted sales and direct materials purchases are as follows: NIU's sales are 40% cash and 60% credit. It collects credit sales 10% in the month of sale, 50% in the month following...
-
Discuss three suggestions for practicing your delivery.
-
What is adverse selection? What is moral hazard? Give an example of these two problems arising between a firm and its suppliers.
-
What is the difference between one-way and two-way ANOVA?
-
Kennedy Company produces a product that sells for $37 per unit and has a variable cost of $22 per unit. Kennedy incurs annual fixed costs of $75,000. Required a. Determine the sales volume in units...
-
Indicate whether each of the following expenditures should be classified as land, land improvements, buildings, equipment, or none of
-
Fort Worth, Inc., specializes in manufacturing some basic parts for sports utility vehicles SUVs) that are produced and sold in the United States. Its main advantage in the United States is that its...
-
Consider the direct heat transfer from a heat reservoir at T 1 to another heat reservoir at temperature T 2 , where T 1 > T 2 > T . It is not obvious why the lost work of this process should depend...
-
Liquids (identified below) at 25C are completely vaporized at 1(atm) in a countercurrent heat exchanger. Saturated steam is the heating medium, available at four pressures: 4.5, 9, 17, and 33 bar....
-
Assume that the consumption schedule for a private open economy is such that consumption C = 50 + 0.8Y. Assume further that planned investment Ig and net exports Xn are independent of the level of...
-
I need help with discussion posts that respond to 3 of these comments. 2 of them being the first on each picture. RUBRIC: articles to mention Coleman, R., & Banning, S. (2006). Network TV news'...
-
2. Best Use of Scarce Resource DigiCom Corporation produces three sizes of television sets: 12-inch screen, 26-inch screen, and 40-inch screen. Revenue and cost information per unit for each product...
-
Gunther invested $15,000 into a segregated fund with a 65% maturity guarantee 10 years ago. The fund is now maturing and has a current market value of $22,261. Gunther decides to withdraw his...
-
(a) Consider the following financial data (in millions of dollars) for Costello Laboratories over the period of 2014-2018: Year Sales Net income Total assets Common equity 2014 $3,800 $500 $3,900...
-
The Pizza Pie 'N Go sells about 2300 one-topping pizzas each month. The circle graph displays the most requested one-topping pizzas, by percentage, for one month. Most Popular One-Topping Pizzas...
-
What is a substitution right, and when does that right result in a contract not being a lease?
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
A refrigeration system cools a brine from 25oC to -15 C at the rate 20 kg s-1. Heal is discarded to the atmosphere at a temperature of 30'C. What is the power requirement if the thermodynamic...
-
An electric motor under steady load draws 9.7 amperes al 110 volts; it delivers 1.25(hp) of mechanical energy. The temperature of the surroundings is 300 K. What is the total rate of entropy...
-
A 25-ohm resistor at steady state draws a current of 10 ampere`s, Its temperature is 310 K; the temperature of the surroundings is 300 K. What is the total rate of entropy generation SG? What is its...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


