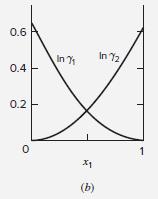
Table 13.10 gives values of parameters for the Wilson equation for the acetone(1)/methanol(2) system. Estimate values of
Question:
Table 13.10 gives values of parameters for the Wilson equation for the acetone(1)/methanol(2) system. Estimate values of ln γ∞1 and ln γ∞2 at 50°C. Compare with the values suggested by Fig. 13.4(b). Repeat the exercise with the NRTL equation.
Table 13.10
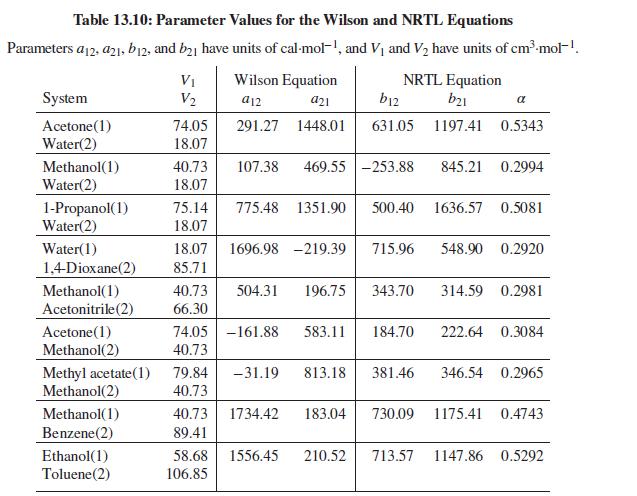
Fig. 13.4(b)
Transcribed Image Text:
Table 13.10: Parameter Values for the Wilson and NRTL Equations Parameters a12, a21, b12, and ba1 have units of cal-mol-!, and Vị and V2 have units of cm mol-!. Wilson Equation NRTL Equation b12 VI System V2 a12 b21 a 74.05 18.07 Acetone(1) 291.27 1448.01 631.05 1197.41 0.5343 Water(2) 469.55 -253.88 Methanol(1) Water(2) 40.73 107.38 845.21 0.2994 18.07 75.14 775.48 1351.90 1-Propanol(1) Water(2) 500.40 1636.57 0.5081 18.07 Water(1) 18.07 1696.98 -219.39 715.96 548.90 0.2920 1,4-Dioxane(2) 85.71 Methanol(1) 40.73 504.31 196.75 343.70 314.59 0.2981 Acetonitrile(2) 66.30 Acetone(1) 74.05 -161.88 583.11 184.70 222.64 0.3084 Methanol(2) Methyl acetate(1) Methanol(2) 40.73 79.84 -31.19 813.18 381.46 346.54 0.2965 40.73 Methanol(1) 40.73 1734.42 183.04 730.09 1175.41 0.4743 Benzene(2) 89.41 210.52 Ethanol(1) Toluene(2) 58.68 1556.45 713.57 1147.86 0.5292 106.85
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
To estimate the values of ln and ln for the acetone1methanol2 system at 50C using the Wilson and NRTL equations we can use the parameters given in Tab...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Repeat Exercise 13 for the integral In Exercise 13 Determine the values of n and h required to approximate To within 105 and compute the approximation. Use a. Composite Trapezoidal rule. b. Composite...
-
Table B.2 of Appendix B provides parameters for an equation that gives P sat as a function of T for a number of pure compounds. For one of them, determine the heat of vaporization at its normal...
-
Repeat Exercise 1 using four-digit rounding arithmetic, and compare the errors to those in Exercise 3. In Exercise 1 a. b. 0.5 0.4794 0.6 0.5646 0.7 0.6442 x f(x) f'(x) 0.0 0.00000 0.2 0.74140 0.4...
-
How much taxes do Employees and Employers pay in F.I.C.A? a) Employees and Employers paid 1.45% in taxes. b) Employees and Employers paid 7.65% in taxes. c) Employers and Employees paid 2.7% in...
-
Explain how both an emission tax and tradable pollution permits system can reduce pollution.
-
Prepare a skeleton statement of revenues, expenditures, and changes in fund balances using the headings in Illustration 2-11 (e.g., Revenues, Expenditures, and Excess (Deficiency) of Revenues over...
-
If a firm takes steps that increase its expected future ROE, does this necessarily mean that the stock price will also increase? Explain. AppendixLO1
-
Listed are the equity sections of balance sheets for years 2011 and 2012 as reported by Mountain Air Ski Resorts, Inc. The overall value of stockholders equity has risen from $2,000,000 to...
-
Wildhorse Inc. produces three separate products from a common process costing $100,500. Each of the products can be sold at the split-off point or can be processed further and then sold for a higher...
-
Go to Case 7.3, Sutton v. Eastern New York Youth Soccer Association, Inc. Read the excerpt and answer the following questions. (a) Issue: The focus in this case was the application of the doctrine of...
-
Consider the following model for G E RT of a binary mixture: This equation in fact represents a family of two-parameter expressions for G E RT; specification of k leaves A 12 and A 21 as the free...
-
Possible correlating equations for ln 1 in a binary liquid system are given here. For one of these cases, determine by integration of the Gibbs/Duhem equation [Eq. (13.11)] the corresponding...
-
Compute the area between the following curves. Illustrate your answers. a. f(x) = x 3 and g(x) = x 2 bounded between x = 0 and 0.5. b. f(x) = x 3 and g(x) = x 2 bounded between x = 1 and 5.
-
List and describe three common interview mistakes which compromise the quality of the selection process.
-
Player I chooses a positive integer x > 0 and player II chooses a positive integer y > 0. The player with the lower number pays a dollar to the player with the higher number unless the higher number...
-
1. Distinguish between risk management and risk transfer. As the manager of XYZ FBO, what measures would you put in place to ensure risk reduction?
-
Devlop a sumary of your understanding of Learning and Retention. In the sumary, think about how you see Learning and Retention fitting into the entire study of Organizational Behavior. How does the...
-
Emani, Peters and Desai et al (2018) "conducted a cross-sectional survey of adopters and non-adopters of the portal...the survey consisted of perceived attributes from the DOI theory,...
-
Selected hypothetical comparative statement data for the giant bookseller Barnes & Noble are presented here. All balance sheet data are as of the end of the fiscal year (in millions). Instructions...
-
The National Collegiate Athletic Association (NCAA) and the National Federation of State High School Associations (NFHS) set a new standard for non-wood baseball bats. Their goal was to ensure that...
-
The virial coefficient can be related to the pair potential by Eqn. 7.59. (a) Derive the integrated expression for the second virial coefficient in terms of the square well potential parameters /k, ,...
-
What forms does the derivative (C V /V) T have for a van der Waals gas and a Redlich- Kwong gas? Comment on the results.
-
Develop an expression for the Gibbs energy departure function based on the Redlich- Kwong (1958) equation of state: Z = 1 + bp ap 1-bp RT/(1+bp)
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


