The energy equation for mixtures can be written for polymers in the form: By analogy to the
Question:
The energy equation for mixtures can be written for polymers in the form: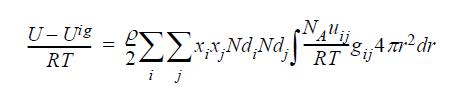
By analogy to the development of the Scatchard-Hildebrand theory, this can be rearranged to: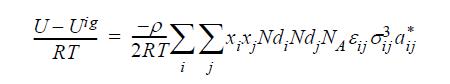
where
Ndi = degree of polymerization for the ith component
ρ = molar density
xi = mole fraction of the ith component
NA = Avogadro¡¦s number
U = molar internal energy.
aii* = 3 + 2/Ndi
aij* = aii* ajj*)1/2
σij3 = (σi3 + σi3 )/2
εij = (εii εjj)1/2
Derive an expression for ln γ1 for the activity coefficient model presented above.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





