The ESD equation of state is given by P = b, c is a shape parameter
Question:
The ESD equation of state is given by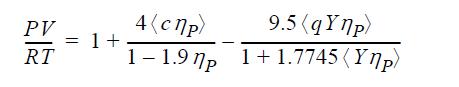
ηP = bρ, c is a “shape parameter” which represents the effect of non-sphericity on the repulsive term, and q = 1 + 1.90476(c - 1). A value of c = 1 corresponds to a spherical molecule. Y is a temperature-dependent function whose role is similar to the temperature dependence of the a parameter in the Peng-Robinson equation. Use the methods of Example 7.7 to fit b and Y to the critical point for ethylene using c = 1.3.
Transcribed Image Text:
PV RT = 1 + 4(cnp) 1-1.97p 9.5 (qYnp) 1+1.7745(Yp)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To find the values of Z use the procedure given in Example 77 In order to determine A and B for the van der Waals equation we must first remember the ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
A friend studies a first-order reaction and obtains the following three graphs for experiments done at two different temperatures. (a) Which two graphs represent experiments done at the same...
-
The ESD equation is presented in problem 7.19. Derive expressions for the enthalpy and entropy departure functions in terms of this equation of state. Data from Problem 7.19 The ESD equation of state...
-
Write the Lewis structure for CHCHCHO and determine the number of sigma and pi bonds in the molecule. Sigma bonds : Pi bonds:
-
Prove that e is an irrational number using the following argument by contradiction. Suppose that e = M/N, where M, N are nonzero integers. (a) Show that M!e- is a whole number. (b) Use the power...
-
Four roommates are planning to spend the weekend in their dorm room watching old movies, and they are debating how many to watch. Here is their willingness to pay for each film: a. Within the dorm...
-
Describe how managers should deal with problematic behavior.
-
From words to probabilities. Probability is a measure of how likely an event is to occur. Match one of the probabilities that follow with each statement of likelihood given. (The probability is...
-
Modco was founded in 1960, with the opening of the first Modco discount store, and was incorporated as Modco Stores Inc. in January 1970. The companys shares were listed on the NYSE in 1975. Modco...
-
A company that prepares its financial statements according to International Financial Reporting Standards (IFRS) accounts for software development costs by: Multiple Choice expensing those costs in...
-
A molecular simulation sounds like an advanced subject, but it is really quite simple for hard spheres. Furthermore, modern software is readily available to facilitate performing simulations, after...
-
Consider the equation of state where P = b/V. The first term on the right-hand side is known as the Carnahan-Starling equation for the hard-sphere compressibility factor. (a) Determine the...
-
A charge of 4.0 10-6 C is placed on a small conducting sphere that is located at the end of a thin insulating rod whose length is 0.20 m. The rod rotates with an angular speed of = 150 rad/s about...
-
Identify at least two business systems that support the development of effective work relationships Briefly explain how each system supports the development of effective work relationships.
-
Power and Influence Personal Plan - How will you navigate the realms of power and influence? Why is this personal plan important for you? What do you want to achieve? do a table with SMART goals -...
-
A single-stage trickling-filter plant is proposed for treating a dilute wastewater with a BOD concentration of 170 mg/L. The plant is located in a warm climate, and the minimum wastewater temperature...
-
For the first assignment for this course, compose a written document that contains the following: A description and assessment of your past experiences with policy and program planning, either your...
-
What are the key motivators driving consumer purchasing decisions in our industry? How do consumers perceive our brand compared to competitors, and what factors influence brand loyalty?
-
In 2019, its first year of operations, Regal Department Store sells $250,000 of gift certificates redeemable for store merchandise that expire one year after their issuance. With a high degree of...
-
1. Firms may hold financial assets to earn returns. How the firm would classify financial assets? What treatment will such financial assets get in the financial statements in accordance with US GAAP...
-
Water was drained from a buret between the 0.12- and 15.78-mL marks. The apparent volume delivered was 15.78 - 0.12 =15.66 mL. Measured in the air at 22C, the mass of water delivered was 15.569 g....
-
Reproduce the spreadsheet in Figure 2-23 and the graph in Figure 2-24. Figure 2-24 Density of Water 1.000 0.998 0.996 - 0.994 0.992 10 20 30 40 Temperature (C) Density (g/mL)
-
Reproduce the spreadsheet in Figure 2-23 and the graph in Figure 2-24. Figure 2-23 Figure 2-24 A E F G H Calculating Density of H2O with Equation 2-4 (from the delightful book by Dan Harris) 3...
-
The star Mira is 1.2 times the mass of the Sun and about 10,000 times more luminous than the Sun. Would Mira fit into the table above? Why or why not?
-
Which of the following was one of the most valuable benefits a company received as a sponsor of NHL games?
-
Cinder Inc. is a Canadian-controlled private corporation based in your province. The company operates a wholesale business. The following information is provided for its year ended May 31, 2023: Net...

Study smarter with the SolutionInn App


