The pressure above a mixture of chloroform and tetrahydrofuran at 50C is measured to be 52 kPa.
Question:
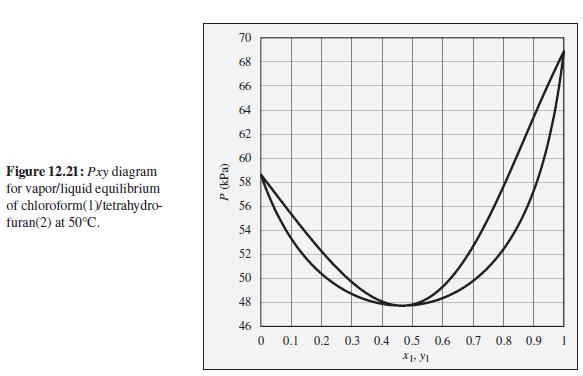
The pressure above a mixture of chloroform and tetrahydrofuran at 50°C is measured to be 52 kPa. What are the possible compositions of the liquid and vapor phases?
To the Pxy diagram for chloroform(1)/tetrahydrofuran(2) at 50°C shown in Fig. 12.21.
Transcribed Image Text:
70 68 66 64 62 60 Figure 12.21: Pxy diagram for vapor/liquid equilibrium of chloroform(1Vtetrahydro- furan(2) at 50°C. 58 56 54 52 50 48 46 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 P (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
A relativistic rocket is measured to be 50 m long, 2.5 m high, and 2.0 m wide by its pilot. It is traveling at 0.65c (in the direction parallel to its length) relative to an inertial observer. (a)...
-
A mixture of chloroform and tetrahydrofuran is heated in a closed system at 120 kPa to a temperature of 75C, and two phases are observed to be present. What are the possible compositions of the...
-
The pressure of an automobile tire is measured to be 190 kPa (gage) before a trip and 215 kPa (gage) after the trip at a location where the atmospheric pressure is 95 kPa. If the temperature of air...
-
Jay Bhattacharya and Kate Bundorf of Stanford University have found evidence that people who are obese and who work for firms that provide health insurance receive lower wages than workers at those...
-
1. Define consumers surplus. 2. Define producers surplus. 3. Explain how trade is most commonly restricted. 4. Explain how tariffs affect consumers and producers surplus. 5. Explain how quotas affect...
-
Myers Business Systems is evaluating the introduction of a new product. The possible levels of unit sales and the probabilities of their occurrence are given next: a. What is the expected value of...
-
Define the following terms: new expansion project and replacement project. AppendixLO1
-
Kincaid Company sells flags with team logos. Kincaid has fixed costs of $639,600 per year plus variable costs of $4.20 per flag. Each flag sells for $12.00. Requirements 1. Use the equation approach...
-
Chemy (The following information applies to the questions displayed below) View previous tem Greg's Bicycle Shop has the following transactions related to its top-selling Mongoose mountain bike for...
-
Refer to the Fundamental Principles governing an audit. Under the Responsibilities Principle, auditors are required to exercise due care and maintain professional skepticism throughout the audit....
-
Consider a binary (two-species) system in vapor/liquid equilibrium. Enumerate all of the combinations of intensive variables that could be fixed to fully specify the intensive state of the system.
-
The pressure above a mixture of chloroform and tetrahydrofuran at 50C is measured to be 62 kPa. What are the possible compositions of the liquid and vapor phases? To the Pxy diagram for...
-
Lab Industries, Inc., issued $50,000 of bonds, paid cash dividends of $8,000, sold long-term investments for $12,000, received $5,000 of dividend revenue, purchased treasury stock for $15,000, and...
-
Write a java program that contain two overloaded methods that accepts two numbers or two characters representing a range example (11, 37) or (c, w) inputted by the user. The method generates a random...
-
Maggie could not conceive a child using natural means, so she sought out a woman who would donate an egg to be surgically implanted in Maggie. Which of the following items are deductible by Maggie in...
-
M corporation is subject to tax only in state b state b law provides for the use of federal taxable income before net operating loss and special deductions as the starting point for computing state...
-
Use Routh Criteria to determine the values of K needed for the system represented by the Characteristic Equation to be stable. (1 + K)s + (2K + 3)s + 2 3K = 0 Obtain the root locus plot for the...
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
The shareholders equity section of Gaines Industriess balance sheet appeared as follows at December 31, 2018: During 2019, the following chronological transactions were recorded: 1. Gaines issued...
-
Find the work done in pumping all the oil (density S = 50 pounds per cubic foot) over the edge of a cylindrical tank that stands on one of its bases. Assume that the radius of the base is 4 feet, the...
-
(a) What is the enthalpy change needed to change 3 kg of liquid water at 0C to steam at 0.1 MPa and 150C? (b) What is the enthalpy change needed to heat 3 kg of water from 0.4 MPa and 0C to steam at...
-
The energy balance can be developed for just about any process. Since our goal is to learn how to develop model equations as well as to simply apply them, it is valuable practice to obtain the...
-
One mole of an ideal gas (C p = 5R/2) in a closed piston/cylinder is expanded from T i = 500 K, P i = 0.6 MPa to P f = 0.1 MPa by the following pathways. For each pathway, calculate U, H, Q, and W EC...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


