The pressure above a mixture of ethanol and ethyl acetate at 70C is measured to be 86
Question:
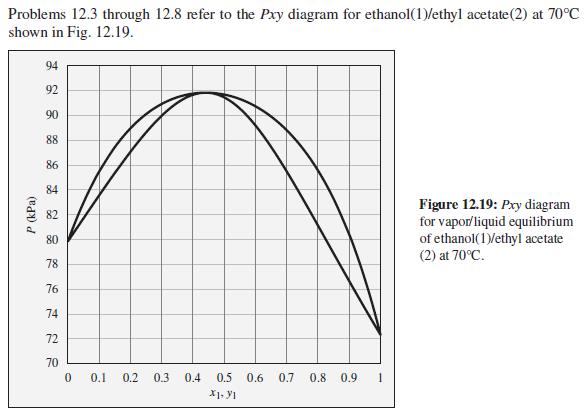
The pressure above a mixture of ethanol and ethyl acetate at 70°C is measured to be 86 kPa. What are the possible compositions of the liquid and vapor phases?
Transcribed Image Text:
Problems 12.3 through 12.8 refer to the Pxy diagram for ethanol(1)/ethyl acetate(2) at 70°C shown in Fig. 12.19. 94 92 90 88 86 84 Figure 12.19: Pxy diagram for vapor/liquid equilibrium of ethanol(1)/ethyl acetate (2) at 70°C. 82 80 78 76 74 72 70 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 P (kPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
To determine the possible compositions of the liquid and vapor phases we need to use a phase equilibrium calculation based on the given pressure and t...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
A relativistic rocket is measured to be 50 m long, 2.5 m high, and 2.0 m wide by its pilot. It is traveling at 0.65c (in the direction parallel to its length) relative to an inertial observer. (a)...
-
A mixture of ethanol and 1-propanol behaves ideally at 36C and is in equilibrium with its vapor. If the mole fraction of ethanol in the solution is 0.62, calculate its mole fraction in the vapor...
-
The pressure of an automobile tire is measured to be 190 kPa (gage) before a trip and 215 kPa (gage) after the trip at a location where the atmospheric pressure is 95 kPa. If the temperature of air...
-
1. Suppose that a stoichiometric mixture of isooctane (C8H18) and air is burned in an engine and then the fuel is changed to 10% (by liquid volume) ethanol and 90% by liquid volume isooctane. If the...
-
The lowest fifth of income earners have a 10 percent income share; the second fifth, a 17 percent income share; the third fifth, a 22 percent income share; the fourth fifth, a 24 percent income...
-
Why are the final figures from the prior years audit included in a working trial balance or lead schedule?
-
Rick, who is single, has been offered a position as a city landscape consultant. The position pays $125,000 in cash wages. Assume Rick has no dependents. Rick deducts the standard deduction instead...
-
The following stockholders equity accounts arranged alphabetically are in the ledger of Crivello Corporation at December 31, 2014. Common Stock ($3 stated value) ................. $2,400,000 Paid-in...
-
"I know headquarters wants us to add that new product line, said Dell Havasi, manager of Billings Company's Office Products Division. But I want to see the numbers before I make any move. Our...
-
For the following project, 3 sections are expected every week. Each week is 5 working days with 8 hours a day. Find the value of X1? Find the value of X2? Find the value of X3? Find the value of X4?...
-
Of the following binary liquid/vapor systems, which can be approximately modeled by Raoults law? For those that cannot, why not? Table B.1 (App. B) may be useful. (a) Benzene/toluene at 1(atm). (b)...
-
A stream of 12 kgs 1 of Cu(NO 3 ) 2 6H 2 O and a stream of 15 kgs 1 of water, both at 25C, are fed to a tank where mixing takes place. The resulting solution passes through a heat exchanger that...
-
The speed of the memory system affects the designers decision on the size of the cache block. Which of the following cache designer guidelines are generally valid? 1. The shorter the memory latency,...
-
You must select an orifice meter for measuring the flow rate of an organic liquid ( $\mathrm{SG}=0.8$, $\mu=15 \mathrm{cP}$ ) in a $4 \mathrm{in}$. sch 40 pipe. The maximum flow rate anticipated is...
-
A team of designers was given the task of reducing the defect rate in the manufacture of a certain printed circuit board. The team decided to reconfigure the cooling system. A total of 973 boards...
-
Level 98%: x1 = 49, n1 = 74, x2 = 62, n2 = 153 In Exercises 712, construct the confidence interval for the difference p1 p2 for the given level and values of x1, n1, x2, and n2.
-
Let X be a continuous random variable with the following PDF Find the MGF of X, M X (s). fx(x) = +) == 12e-1|2|1 e-A/).
-
The number of hours spent studying per day by a sample of 28 students In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 2 8 7 2 261 82 35 37 25 20 73 83...
-
The following three accounts appear in the general ledger of Beiber Corp. during 2022. Instructions From the postings in the accounts, indicate how the information is reported on a statement of cash...
-
Recall that Chapter 8 described the binary search algorithm for finding a particular entry in an ordered list. The idea behind binary search is to begin looking in the exact center of the list. If...
-
Data from the Bureau of Standards (J. Phys. Chem. Ref. Data, vol. 11, suppl. 2, 1982) include the following heats of formation for 1 mol of C a Cl 2 in water at 25C: From these data prepare a plot of...
-
The standard heat of reaction H for gas-phase reactions is independent of the choice of standard-state pressure P. (Why?) However, the numerical value of G for such reactions does depend on P. Two...
-
Equilibrium at 425 K and 15 bar is established for the gas-phase isomerization reaction n-C 4 H 10 (g) iso-C 4 10 (g) Estimate the composition of the equilibrium mixture by two procedures: (a)...
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


