Work problem 7.18, then obtain an expression for the fugacity. Determine the value of c (+/- 0.5)
Question:
Work problem 7.18, then obtain an expression for the fugacity. Determine the value of c (+/- 0.5) that best represents the vapor pressure of the specified compound below. Use the shortcut vapor pressure equation to estimate the experimental vapor pressure for the purposes of this problem.
(a) CO2
(b) Ethane
(c) Ethylene
(d) Propane
(e) n-Hexane
Data from problem 7.18
Consider the equation of state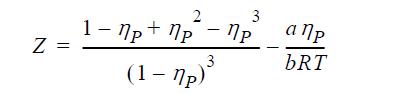
where ηP = b/V. The first term on the right-hand side is known as the Carnahan-Starling equation for the hard-sphere compressibility factor.
Determine the relationships between a, b, c and Tc , Pc, Zc.
What practical restrictions are there on the values of Zc that can be modeled with this equation?
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





