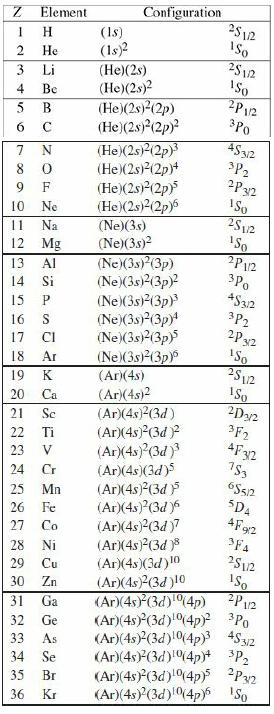
Question: (a) Figure out the electron configurations (in the notation of Equation 5.44) for the first two rows of the Periodic Table (up to neon), and
(a) Figure out the electron configurations (in the notation of Equation 5.44) for the first two rows of the Periodic Table (up to neon), and check your results against Table 5.1.
(b) Figure out the corresponding total angular momenta, in the notation of Equation 5.45, for the first four elements. List all the possibilities for boron, carbon, and nitrogen.
Equation 5.44
![]()
Equation 5.45
![]()
Table 5.1
Ground-state electron configurations for the first four rows of the Periodic Table.
(1s) (2s) (2p) (5.44)
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
a b Hydrogen 1s helium 1s2 lithium 1s22s be... View full answer

Get step-by-step solutions from verified subject matter experts


