3 moles of pure water are adiabatically mixed with 1 mole of pure ethanol at a constant...
Question:
3 moles of pure water are adiabatically mixed with 1 mole of pure ethanol at a constant pressure of 1 bar. The initial temperatures are the pure components are equal. If the fi nal temperature is measured to be 311.5 K, determine the initial temperature. The enthalpy of mixing between water (1) and ethanol (2) has been reported to be fi t by: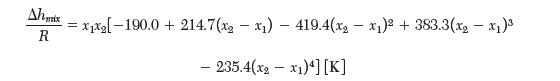
Transcribed Image Text:
Ahmix R =x[-190.0+ = 214.7(x-x) 419.4(x-x) + 383.3(x - x) - 235.4(x-x1)4] [K]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
A stream of pure water fl owing at 1 mol/s is adiabatically mixed with a stream containing equamolar amounts of water and ethanol, also fl owing at 1 mol/s. This steady-state process occurs at a...
-
Mays and McCovey are beer-brewing companies that operate in a duopoly (two-firm oligopoly). The daily marginal cost (MC) of producing a can of beer is constant and equals $0.80 per can. Assume that...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Calculate the binding energy per nucleon for a 14/7N nucleus.
-
Using data from the U.S. Census Bureau and other sources, www.nerdwallet.com estimated that considering only the households with credit card debts, the average credit card debt for U.S. households...
-
Table 181 shows the duration calculation for a 5 percent, fiveyear bond. The bond is priced at $974.17 because interest rates have risen so that the current Yield to Maturity (YTM) is 5.6 percent...
-
Identify the types of selection errors and biases managers must overcome when interviewing job applicants. (pp. 137-140)
-
Several years ago, Blaha Company purchased Husker Company as a subsidiary. At that time, Blaha Company recorded goodwill of $100,000 related to the purchase. Since that time, the company has not...
-
b) Laura's utility-of-wealth function is u(w) = 1 - w Her current wealth is 100. There is a probability of 0.2 that 60 of her wealth will be destroyed in an accident. Insurance can be purchased for...
-
1. Which process should VBB choose to produce?? 2. How much would VBP be willing to pay for the testing that is currently offered, for each batch?? 3. Would we be considered a perfect test, at twice...
-
Fit the data in Problem 6.53 to the form: (a) Use the analytical method to come up with an expression of the partial molar volume of ethanol. (b) Determine the values at x1 = 0.8, x1 = 0.4, and x1 =...
-
Repeat Example 5.4 using the LeeKesler generalized correlation data for enthalpy departure to account for nonideal behavior.
-
Problem refer to the following Venn diagram. Which of the numbers x, y, z, or w must equal 0 if A and B are disjoint? A B y
-
Based on a survey, assume that 42% of consumers are comfortable having drones deliver their purchases. Suppose that we want to find the probability that when six consumers are randomly selected,...
-
What is the social location that determines this speech community? Is it determined by race, class, gender, sexuality, or some other social location? What makes this speech community unique? What are...
-
Write a program named SumOfNumberOfSquares.java that prompts user to enter a number of integers and calculates the sum of their squares. The following is a sample run. The green fonts represent user...
-
6.4 Charles Augustin de Coulomb was a French physicist who is best known for formulating the law that calculates the force between two electric charges. To honor Coulomb, the unit of electric charge...
-
What amount of cash payments to suppliers will be reported by Indigo Company for the year ended December 31, 2024?
-
Go through your daily newspaper and identify: (a) Seven or eight news items of an international economic character; (b) The importance or effect of each of these problems on the U.S. economy; (c) The...
-
A number of years ago the United Food and Commercial Workers Union organized 800 workers of the 1035 employees at one of the Wilson Brothers food operations in Toronto, Ontario. The employees include...
-
How would the energy versus dihedral angle plot for 2-methylpropane (isobutene) differs from that for propane?
-
Draw an energy versus dihedral angle plot for the conformations of 2, 3-dirnethylbutane about the C-2C-3 bond.
-
Discuss the geometry and the types of strain present in these compounds: (a) Cyclopropane (b) Cyclobutene (c) Cyclopentane (d) Cyclohexane (e) Cyclodecane
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


