A binary mixture of solids a and b is known to form three distinct solid phases: a,
Question:
A binary mixture of solids a and b is known to form three distinct solid phases: a, b and g.
Gibbs energy is plotted vs. mole fraction a for the two systems shown on the next page. Each of these plots is made at constant temperature and pressure. For each system, describe the phases that are present and their composition for the entire range of mole fraction a. Explain.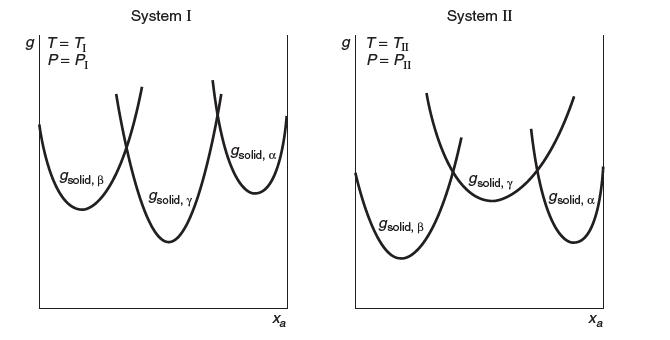
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





