At what temperature does water boil on the top of Mount Everest, elevation z = 8848 m?
Question:
At what temperature does water boil on the top of Mount Everest, elevation z = 8848 m?
Recall that the dependence of pressure with altitude is given by: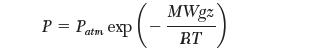
where, Patm is atmospheric pressure, g is the gravitational acceleration, and MW is the molecular weight of the gas.
Transcribed Image Text:
P = Patm exp MWgz RT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Answer Step 1 Given Dependence of pressure with altitude PP exp Mwgz RT At z8848 m Find Temperature ...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Gaseous hydrogen contained initially under standard conditions in a sealed vessel of volume V = 5.0 1 was cooled by T = 55 K. Find how much the internal energy of the gas will change and what amount...
-
The average atmospheric pressure on earth is approximated as a function of altitude by the relation Patm = 101.325 (1 = 0.02256z 5.256, where Patm is the atmospheric pressure in kPa and z is the...
-
The average atmospheric pressure on earth is approximated as a function of altitude by the relation Patm = 101.325 (1 - 0.02256z)5.256, where Patm is the atmospheric pressure in kPa and z is the...
-
For brands is it more important to create big idea that is required to drive a customer connection or can it be stifled by corporate requirements to measure performance with automation and generate...
-
What is the purpose of a bilateral income tax treaty between two countries?
-
On December 31, 2022, the equity accounts of Book Creations, Inc., contained the following balances: Common stock ($10 par, 100,000 shares authorized) 50,000 shares issued and outstanding $500,000...
-
Assume a firm faces an S-shaped sales response function. What happens to the ratio of incremental sales revenue to incremental marketing effort at the (a) bottom, (b) middle, and (c) top of this...
-
A large retailer reported revenue of $1,665,000. The companys gross profit percentage was 44 percent. What amount of cost of goods sold did the company report?
-
A stock price has an expected return of 9% and a volatility of 25%. It is currently $40. What is the probability that it will be less than $30 in 18 months? Please show 'step by step'. I keep getting...
-
Water is cooled in a rigid closed container from the critical point to 10 bar. Determine the quality of the fi nal state.
-
The Reamur temperature scale uses the normal freezing and boiling points of water to defi ne 0and 80, respectively. What is the value of room temperature (22C) on the Reamur scale?
-
What are the key causes of reduced productivity and/or commitment in an organisation with which you are familiar? To what extent do you think that these issues will be improved or resolved by the...
-
Idenfity whether the following book - tax adjustments are permanent or temporary differences. ( a ) Federal Income Tax Expense ( b ) Depreciation Expense ( c ) Accrued Compensation ( d ) Dividends...
-
2 . ) Pozycki, LLC has reported losses of $ 1 0 0 , 0 0 0 per year since its founding in 2 0 1 6 . For 2 0 2 3 , Pozycki anticipates a profit of about $ 1 0 0 , 0 0 0 . There are 3 equal members of...
-
Elena is a single taxpayer for tax year 2023. On April 1st, 2022, Elena's husband Nathan died. On July 13, 2023, Elena sold the residence that Elena and Nathan had each owed and used as their...
-
Rodriguez Corporation issues 12,000 shares of its common stock for $56,600 cash on February 20. Prepare journal entries to record this event under each of the following separate situations. 1. The...
-
Problem 3: A large rectangular plate is loaded in such a way as to generate the unperturbed (i.e. far-field) stress field xx = Cy; yy = -C x; Oxy = 0 The plate contains a small traction-free circular...
-
Contrast lossless and lossy compression.
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
Suggest a structure for the product of nucleophilic substitution obtained on Solvolysis of tert-butyl bromide in methanol, and outline a reasonable mechanism for its formation.
-
Identify the compound in each of the following pairs that reacts at the faster rate in an SN1 reaction: (a) Isopropyl bromide or isobutyl bromide (b) Cyclopentyl iodide or 1-methylcyclopentyl iodide...
-
Identify the compound in each of the following pairs that reacts with sodium iodide in acetone at the faster rate: (a) 1-Chlorohexane or cyclohexyl chloride (b) 1-Bromopentane or 3-bromopentane (c)...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


