Calculate the enthalpy of reaction at 298 K for the following reactions: (a) CH4(g) + 20(g) CO(g)
Question:
Calculate the enthalpy of reaction at 298 K for the following reactions: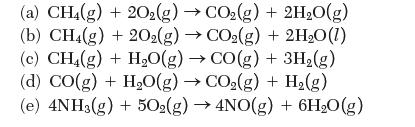
Transcribed Image Text:
(a) CH4(g) + 20(g) CO(g) + 2HO(g) (b) CH4(g) + 2O2(g) CO(g) + 2HO(1) (c) CH4(g) + HO(g) CO(g) + 3H(g) (d) CO(g) + HO(g) CO(g) + H(g) (e) 4NH3(g) + 502(g) 4NO(g) + 6H0(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)

Answered By

RAHUL SINGH SOUN
I have qualified gate, this makes me technically sound.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Vehicle air bags protect passengers by using a chemical reaction that generates gas rapidly. Such a reaction must be both spontaneous and explosively fast. A common reaction is the decomposition of...
-
The Haber Bosch Process is a critically important chemical reaction run on an industrial scale. The reaction is shown below: 3 H+N2 =2 NH3 a) Using the thermodynamic tables in your textbook determine...
-
The molecule methylamine (CH3NH2) can act as a monodentate ligand. The following are equilibrium reactions and the thermochemical data at 298 K for reactions of methylamine and en with Cd2+ (aq); (a)...
-
Question 4 Tic-tac-toe (also known as noughts and crosses) is a game for two players, X and O, who take turns marking the spaces in a 3x3 grid. The player who succeeds in placing three of their marks...
-
Assume Mess stock has a beta of 1.2. If the risk-free rate is 7 percent, and the market return is 10 percent, what is the expected return on Mess stock?
-
Which of the following would indicate an improvement in a companys financial position, holding other things constant? The times-interest-earned ratio declines The inventory and total assets turnover...
-
Find the probability that a student had a score between 95 and105. Is this an unusual event? Explain.
-
The Metro Central Railroad is a commuter railroad that stops at the Village of Katonah. Katonah, a growing community, decides to construct and operate a parking lot near the railroad station to...
-
Exercise 8-3 Direct Materials Budget [LO8-4) Two grams of musk oil are required for each bottle of Mink Caress, a very popular perfume made by a small company in western Siberia. The cost of the musk...
-
Calculate the adiabatic fl ame temperature of acetylene gas at a pressure of 1 bar under the following conditions. The reactants are initially at 298 K. Assume that the acetylene reacts completely to...
-
One mole of saturated liquid propane and 1 mole of saturated vapor are contained in a rigid container at 0C and 4.68 bar. How much heat must be supplied to evaporate all of the propane. At 0C, You...
-
Kingery Corporation owns a machine that is fully depreciated but is still being used. How should Kingery account for this asset and report it in the financial statements?
-
At the Business Level there are a couple main strategies that companies use- Cost Leadership and Differentiation. What is the difference between them? Share some examples of companies or specific...
-
https://youtu.be/c_Eutci7ack After watching the video, what are your thoughts on Power? Would you want to have this Power ? Why would you not want this Power? If you are a manager or want to be a...
-
Find anti derivative of ( 2 t - 4 + 3 ^ ( 1 / 2 ) ) / t ^ ( 1 / 2 )
-
How Adidas is using creative narratives to build brand equity Adidas' outdoor division is drawing on the expertise of its wider athletic business while at the same time flexing its creative muscle to...
-
May I have a word" Alysha Stark popped her head in at the corner office of the Managing Director Mike O' Connor. It's early on a Monday morning. When Alysha, his star Director, starts something this...
-
What is Mosaic?
-
Suppose that a company has 10.000 outstanding shares in the beginning of the year. On April 1st, the company increases its shares by 6.000. On July 1st, the company increases its shares again, but...
-
Uscharidin is the common name of a poisonous natural product having the structure shown. Locate all of the following in uscharidin:
-
Write the structural formula of a compound of molecular formula C4H8Cl2 in which (a) All the carbons belong to methylene groups (b) None of the carbons belong to methylene groups
-
Female tiger moths signify their presence to male moths by giving off a sex attractant. The sex attractant has been isolated and found to be a 2-methyl-branched alkane having a molecular weight of...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


